1. In this experiment, you will make a set of solutions containing different concentrations of crystal violet (CV*) by mixing different volumes of a stock CV* solution with water to give final volumes of 3.0 mL. The stock solution of CV+ has a concentration of 2.0 x 10-5 M and is dark purple. The diluted solutions will be prepared in cuvettes. 2.0 x 105 M CV* (purple) H₂O V₁ diluted samples of CV* in cuvettes 3.0 mL= V2 Use c₁V₁= C₂V₂ to calculate the volume of the stock 2.0 x 105 M CV+ AND the volume of water to make a 3.0 mL sample of 7.5 x 10-6 M CV+. Show all of your calculations.
1. In this experiment, you will make a set of solutions containing different concentrations of crystal violet (CV*) by mixing different volumes of a stock CV* solution with water to give final volumes of 3.0 mL. The stock solution of CV+ has a concentration of 2.0 x 10-5 M and is dark purple. The diluted solutions will be prepared in cuvettes. 2.0 x 105 M CV* (purple) H₂O V₁ diluted samples of CV* in cuvettes 3.0 mL= V2 Use c₁V₁= C₂V₂ to calculate the volume of the stock 2.0 x 105 M CV+ AND the volume of water to make a 3.0 mL sample of 7.5 x 10-6 M CV+. Show all of your calculations.
Chapter8: Sampling, Standardization, And Calibration
Section: Chapter Questions
Problem 8.1QAP
Related questions
Question

Transcribed Image Text:1 of 2
- + Automatic Zoom
1. In this experiment, you will make a set of solutions containing different concentrations of
crystal violet (CV) by mixing different volumes of a stock CV* solution with water to give final
volumes of 3.0 mL. The stock solution of CV+ has a concentration of 2.0 x 10-5 M and is dark
purple. The diluted solutions will be prepared in cuvettes.
2.0 x 105 M CV*
(purple)
H₂O
V1
diluted samples of CV*
in cuvettes
3.0 mL= V₂
Use c₁V₁= C₂V₂ to calculate the volume of the stock 2.0 x 105 M CV+ AND the volume of water to
make a 3.0 mL sample of 7.5 x 10-6 M CV+. Show all of
your calculations.
Expert Solution
Step 1
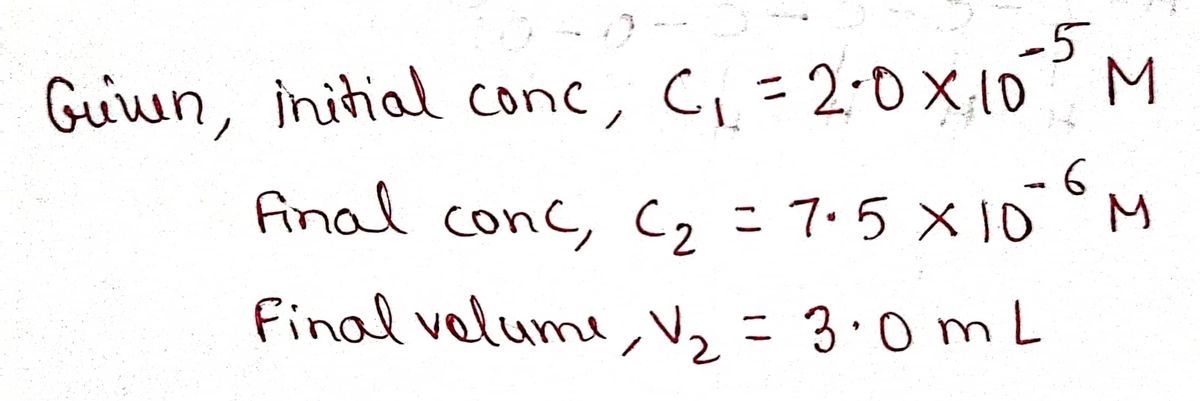
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

