A formula 1 race car averages about 1.25 km/L. Show how to determine and then calculate this mileage in miles/gal using only the exact english to SI conversion factors listed below. (1 inch= 2.54 centimeters, 5280 ft= 1 mile, 1 fl oz. =29.57 ml, 1 gal=128 fl oz.)
A formula 1 race car averages about 1.25 km/L. Show how to determine and then calculate this mileage in miles/gal using only the exact english to SI conversion factors listed below. (1 inch= 2.54 centimeters, 5280 ft= 1 mile, 1 fl oz. =29.57 ml, 1 gal=128 fl oz.)
Chapter1: Matter, Measurements, And Calculations
Section: Chapter Questions
Problem 1.79E
Related questions
Question
A formula 1 race car averages about 1.25 km/L. Show how to determine and then calculate this mileage in miles/gal using only the exact english to SI conversion factors listed below. (1 inch= 2.54 centimeters, 5280 ft= 1 mile, 1 fl oz. =29.57 ml, 1 gal=128 fl oz.)
Expert Solution
Step 1
Coverting km to miles:

Step 2
Converting L to gal.
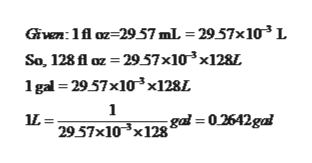
Step by step
Solved in 4 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning


Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning