Granular activated carbon is used to adsorb haloacetic acids from 1L of water. The initial mater concentration of haloacetic acids is 0.9 mg/L. The following data were obtained from an experiment: logg = log k + log C Mass GAC added (g) 0.2 0.5 2.0 5.0 10 20 C, mg/L of haloacetic acids 0.77 0.65 0.32 0.19 0.14 0.09 y = 1.34 (77) +0.018 = 1.158 logg 1=1.34y+0.018 는 logg The Freundlich isotherm analysis by plotting (on y axis) vs. 1/C (on x axis) gives a linear regression line of y = 1.34x + 0.018 with R² of 0.982. Note that q is adsorption density (mass adsorbate/mass adsorbent). What is the required GAC dosage in mg/L to lower the concentration of haloacetic acids to 0.05 mg/L? q = KC we want C=0.05 mg IL
Granular activated carbon is used to adsorb haloacetic acids from 1L of water. The initial mater concentration of haloacetic acids is 0.9 mg/L. The following data were obtained from an experiment: logg = log k + log C Mass GAC added (g) 0.2 0.5 2.0 5.0 10 20 C, mg/L of haloacetic acids 0.77 0.65 0.32 0.19 0.14 0.09 y = 1.34 (77) +0.018 = 1.158 logg 1=1.34y+0.018 는 logg The Freundlich isotherm analysis by plotting (on y axis) vs. 1/C (on x axis) gives a linear regression line of y = 1.34x + 0.018 with R² of 0.982. Note that q is adsorption density (mass adsorbate/mass adsorbent). What is the required GAC dosage in mg/L to lower the concentration of haloacetic acids to 0.05 mg/L? q = KC we want C=0.05 mg IL
Oh no! Our experts couldn't answer your question.
Don't worry! We won't leave you hanging. Plus, we're giving you back one question for the inconvenience.
Submit your question and receive a step-by-step explanation from our experts in as fast as 30 minutes.
You have no more questions left.
Message from our expert:
Our experts are unable to provide you with a solution at this time. Try rewording your question, and make sure to submit one question at a time. We've credited a question to your account.
Your Question:
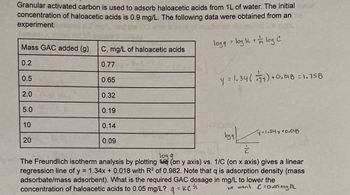
Transcribed Image Text:Granular activated carbon is used to adsorb haloacetic acids from 1L of water. The initial mater
concentration of haloacetic acids is 0.9 mg/L. The following data were obtained from an
experiment:
logg = log k + log C
Mass GAC added (g)
0.2
0.5
2.0
5.0
10
20
C, mg/L of haloacetic acids
0.77
0.65
0.32
0.19
0.14
0.09
y = 1.34 (77) +0.018 = 1.158
logg
1=1.34y+0.018
는
logg
The Freundlich isotherm analysis by plotting (on y axis) vs. 1/C (on x axis) gives a linear
regression line of y = 1.34x + 0.018 with R² of 0.982. Note that q is adsorption density (mass
adsorbate/mass adsorbent). What is the required GAC dosage in mg/L to lower the
concentration of haloacetic acids to 0.05 mg/L? q = KC
we want C=0.05 mg IL
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781259911156
Author:
Raymond Chang Dr., Jason Overby Professor
Publisher:
McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9780078021558
Author:
Janice Gorzynski Smith Dr.
Publisher:
McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Elementary Principles of Chemical Processes, Bind…
Chemistry
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY
