Using the Kf and Kb equations with electrolytes A certain liquid X has a normal boiling point of 110.90 °C and a boiling point elevation constant K=1.66 °C-kg mol -1 .A solution is prepared by dissolving some ammonium sulfate ((NH SO,) in 150. g of X. This solution boils at 112.2 °C. Calculate the mass of (NH) SO, that was dissolved. Round your answer to 2 significant digits. Explanation Check
Using the Kf and Kb equations with electrolytes A certain liquid X has a normal boiling point of 110.90 °C and a boiling point elevation constant K=1.66 °C-kg mol -1 .A solution is prepared by dissolving some ammonium sulfate ((NH SO,) in 150. g of X. This solution boils at 112.2 °C. Calculate the mass of (NH) SO, that was dissolved. Round your answer to 2 significant digits. Explanation Check
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter13: Fundamental Equilibrium Concepts
Section: Chapter Questions
Problem 101E: A 0.010 M solution of the weak acid HA has an osmotic pressure (see chapter on solutions and...
Related questions
Question

Transcribed Image Text:Using the Kf and Kb equations with electrolytes
A certain liquid X has a normal boiling point of 110.90 °C and a boiling point elevation constant K=1.66 °C-kg mol
-1
.A solution is
prepared by dissolving some ammonium sulfate ((NH SO,) in 150.g of X. This solution boils at 112.2 °C. Calculate the mass of
(NH), SO, that was dissolved.
Round your answer to 2 significant digits.
?
Explanation
Check
©2022 McGraw Hill LLC. All Rights Reserved. Terms of Use
Privacy Center |
étv A
Expert Solution
Step 1
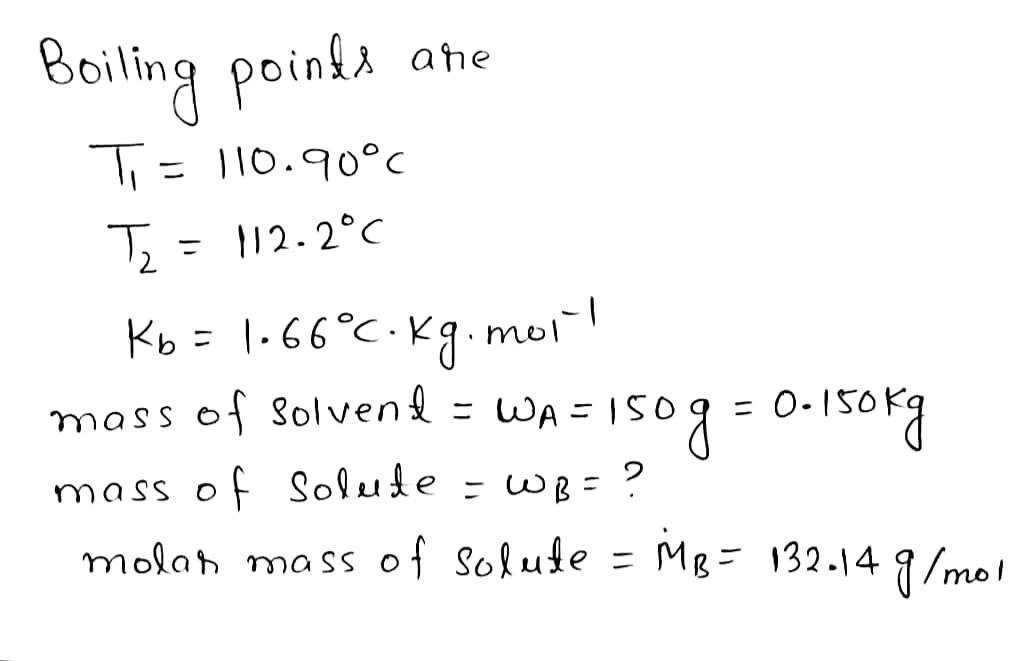
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning