
Prescott's Microbiology
11th Edition
ISBN: 9781260211887
Author: WILLEY, Sandman, Wood
Publisher: McGraw Hill
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 10.4, Problem 1MI
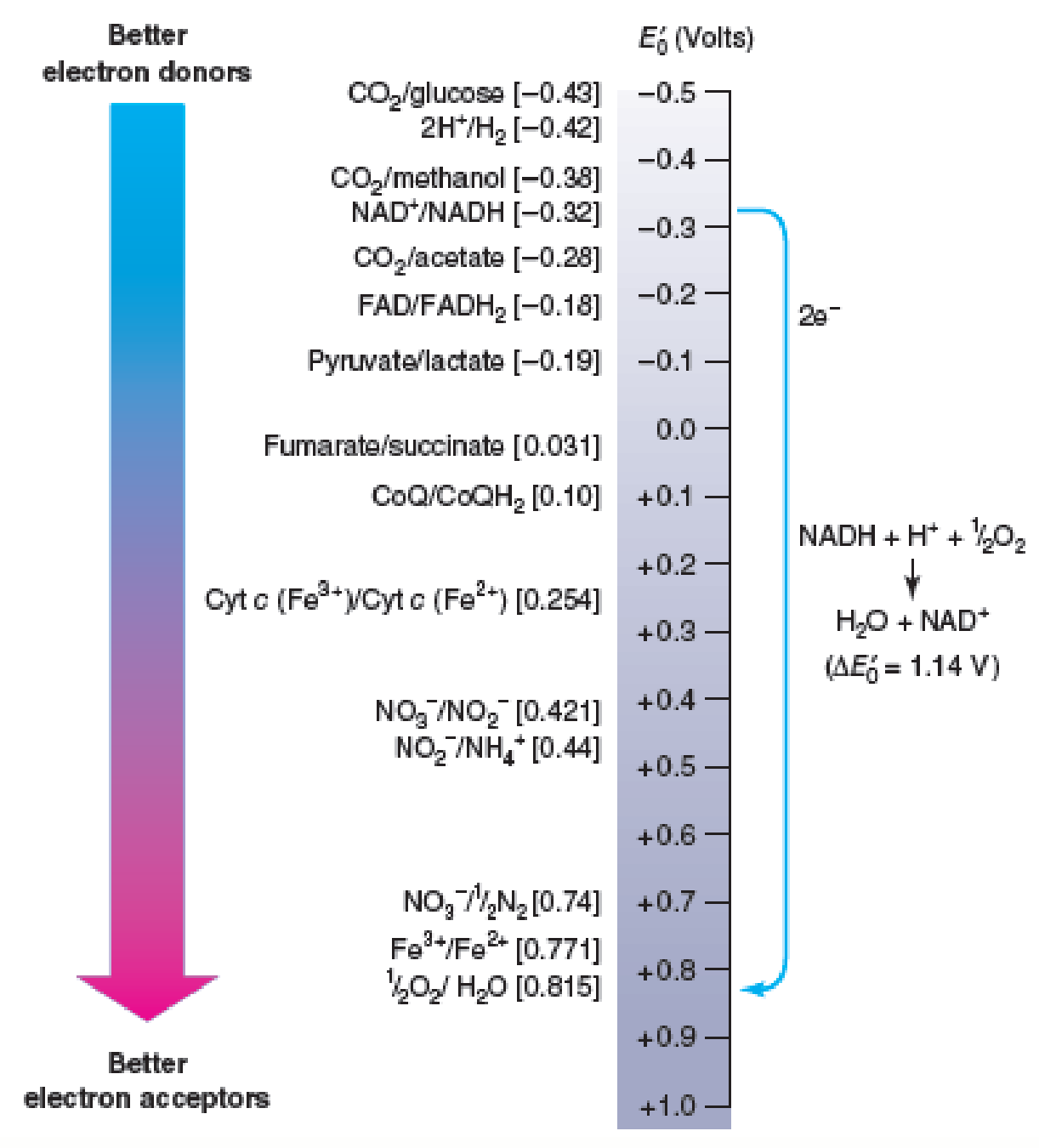
Figure 10.6 Electron Movement and Reduction Potentials. Electrons spontaneously move from donors higher on the tower (more negative potentials) to acceptors lower on the tower (more positive potentials). That is, the donor is always higher on the tower than the acceptor. For example, NADH will donate electrons to oxygen and form water in the process. Some typical conjugate redox pairs are shown on the left, and their reduction potentials are given in brackets.
Refer to figure 10.6 and determine the E′0 for NAD+/NADH and coenzyme Q/CoQH2. Suggest a plausible E′0 value for FMN.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Fo-F1 ATPase. The energy for ATP synthesis from ADP and Pi is provided by the downhill transport of protons through the rotary FoF1 ATP synthase (lecture 22). The enzyme has 3 a-b and 12 ‘c’ subunits. The mitochondrion maintains Df=180 mV (negative inside), pHin = 8, pHout=7, [Pi] = 3 mM and ADP is present as well.
How much energy is available (from the proton electrochemical gradient) for ATP synthesis under these conditions (in kJ/mol)?
What [ATP]/[ADP] ratio will be established at steady-state under these conditions?
What would be the [ATP]/[ADP] ratio if the enzyme had only 9 ‘c’ subunits? Remember that full revolution of the crank (gamma subunit) produces 3 ATP.
NH2
'N.
NH
NH
NH2
Benzamidine and Leupeptin are competitive
trypsin inhibitors. They are shown in their
deprotonated forms at high pH.
Modify the above drawings to show the
protonation and charge at pH 7.0
Draw competitive inhibitors for chymotrypsin
based on these structures
IZ
ZI
ZI
.Intramitochondrial ATP concentrations are about 5 mM, and phosphate con-
centration is about 10 mM. If ADP is five times more abundant than AMP,
calculate the molar concentrations of ADP and AMP at an energy charge
of 0.85. Calculate AG for ATP hydrolysis at 37 °C under these conditions.
The energy charge is the concentration of ATP plus half the concentration of
ADP divided by the total adenine nucleotide concentration:
[ATP] + 1/2[ADP]
[ATP] + [ADP] + [AMP]
Chapter 10 Solutions
Prescott's Microbiology
Ch. 10.1 - Figure 10.2 The Relationship of G to the...Ch. 10.1 - Prob. 1CCCh. 10.1 - Prob. 2CCCh. 10.1 - Prob. 3CCCh. 10.1 - Prob. 4CCCh. 10.2 - Why is ATP called a high-energy molecule? How is...Ch. 10.2 - Describe the energy cycle and ATPs role in it....Ch. 10.3 - Prob. 1MICh. 10.3 - Prob. 2MICh. 10.4 - Figure 10.6 Electron Movement and Reduction...
Ch. 10.4 - How is the direction of electron flow between...Ch. 10.4 - When electrons flow from the NAD+/NADH conjugate...Ch. 10.4 - Which among the following would be the best...Ch. 10.4 - In general terms, how is G related to E0? What is...Ch. 10.4 - Name and briefly describe the major electron...Ch. 10.6 - Will an enzyme with a relatively high Km have a...Ch. 10.6 - Prob. 2MICh. 10.6 - Prob. 1CCCh. 10.6 - Prob. 2CCCh. 10.6 - How does enzyme activity change with substrate...Ch. 10.6 - What special properties might an enzyme isolated...Ch. 10.6 - What are competitive and noncompetitive...Ch. 10.6 - How are enzymes and ribozymes similar? How do they...Ch. 10.7 - Figure 10.19 Allosteric Regulation. The structure...Ch. 10.7 - Prob. 2MICh. 10.7 - Define the terms metabolic channeling and...Ch. 10.7 - Define allosteric enzyme and allosteric effector.Ch. 10.7 - Prob. 3CCCh. 10.7 - Prob. 4CCCh. 10.7 - Prob. 5CCCh. 10 - Prob. 1RCCh. 10 - Prob. 2RCCh. 10 - Prob. 3RCCh. 10 - Examine the structures of macromolecules in...Ch. 10 - Examine the branched pathway shown here for the...Ch. 10 - Prob. 3AL
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- Enzymes catalyze chemical reactions. What constitutes the active site of an enzyme? What are the turnover number (kcat), the Michaelis constant (Km), and the maximal velocity (Vmax) of an enzyme? The kcat (catalytic rate constant) for carbonic anhydrase is 5 × 105 molecules per second. This is a “rate constant,” but not a “rate.” What is the difference? By what oncentration would you multiply this rate constant in order to determine an actual rate of prod- uct formation (V)? Under what circumstances would this rate become equal to the maximal velocity (Vmax) of the enzyme?arrow_forwardA4. what conformational state is stabilized by y in atp synthase? why might achieving this state require energy input from the pmf?arrow_forwardFo-F1 ATPase. The energy for ATP synthesis from ADP and Pi is provided by the downhill transport of protons through the rotary FoF1 ATP synthase . The enzyme has 3 alpha-beta and 12 ‘c’ subunits. The mitochondrion maintains change in membrane potential=180 mV (negative inside), pHin = 8, pHout=7, [Pi] = 3 mM and ADP is present as well. . What [ATP]/[ADP] ratio will be established at steady-state under these conditions? What would be the [ATP]/[ADP] ratio if the enzyme had only 9 ‘c’ subunits? full revolution of the crank (gamma subunit) produces 3 ATP.arrow_forward
- What is the catalytic efficiency of Catalase ? Table. The values of KM and kcat for some Enzymes and Substrates Enzyme Carbonic anhydrase Substrate CO2 HCO3 KM (M) 1.2 x 10-2 2.6 x 10-2 Kcat (s-1) 1.0 x 106 4.0 x 105 Catalase H2O2 2.5 x 10-2 1.0 x 107 Urease Urea 2.5 x 10-2 4.0 x 105 O A. 4 x 108 M-s-1 O B. 4 x 108 M-1.s-1 OC25x 10-9 M-s1 D. 2.5 x 102 M-1.s-1 OE 1.0 x 107 s1arrow_forwardSaccharides: Using the following substrates, estimate the net ATP yield after glycolytic pathway, Kreb’s cycle and electron transport chain. Assume that the estimate for ATP yield per mole of NADH is 3 moles of ATP, while 1 mole of FADH2 is equivalent to 2 moles of ATP, and one mole of GTP is equivalent to one mole of ATP. Show all pertinent solutions and determine: a) ATP used, b) ATP produced, and c) Net ATP. Based on your solutions, rank the substrates based on increasing yield of ATP Two moles of fructose-1,6-biphosphatearrow_forwardSaccharides: Using the following substrates, estimate the net ATP yield after glycolytic pathway, Kreb’s cycle and electron transport chain. Assume that the estimate for ATP yield per mole of NADH is 3 moles of ATP, while 1 mole of FADH2 is equivalent to 2 moles of ATP, and one mole of GTP is equivalent to one mole of ATP. Show all pertinent solutions and determine: a) ATP used, b) ATP produced, and c) Net ATP. Based on your solutions, rank the substrates based on increasing yield of ATP 1. Three moles of glucose-6-phosphate 2. Four moles of pyruvic acidarrow_forward
- a.Write the balanced reactions catalyzed by complex I, II, III, and 1V, and using these, write the balanced net reaction for the electron transport chain. Structures are not necessary. b. Write the balanced reaction for the ATP synthase reaction. c. Write the net reaction for Oxidative phosphorylation showing the ATP produced from NADH oxidation and FADH2 oxidation. (this is in your text) d. Write the balanced reaction for ATP production by aerobic metabolism starting with glucose.arrow_forwardSaccharides: Using the following substrates, estimate the net ATP yield after glycolytic pathway, Kreb’s cycle and electron transport chain. Assume that the estimate for ATP yield per mole of NADH is 3 moles of ATP, while 1 mole of FADH2 is equivalent to 2 moles of ATP, and one mole of GTP is equivalent to one mole of ATP. Show all pertinent solutions and determine: a) ATP used, b) ATP produced, and c) Net ATP. Based on your solutions, rank the substrates based on increasing yield of ATP Five moles of Acetyl coenzyme Aarrow_forwardFigure 1 shows the catalytic triad of a-chymotrypsin. Identify A, B and C. Describe the subsequent steps of stage 1 and stage 2 of a- chymotrypsin mechanism. Illustrate with diagrams. A B CH2 H-N N: H-O Figure 1arrow_forward
- The image below shows ubiquinone. Shaded in yellow is the isoprenoid side chain, which remains unchanged during the electron transport chain. Despite this, what function does it serve? CH3 (CH₂-CH=C-CH₂) 10-H H3CO H3CO I DOO CH3 -- Select one: a. It is hydrophobic, allowing the molecule to be lipid-soluble so that it can diffuse through the membrane between complexe. b. It is hydrophilic, allowing the molecule to be water-soluble and diffuse in the intermembrane space between complexes C. It is hydrophobic, allowing successive molecules to cluster together at complex I d. It is hydrophilic, allowing optimal interaction in the matrix with NADH at complex I Report question issue Notes + 14:27 - COarrow_forward1 = 2, for sufficiently large values of 1. The energetic equivalent of two molecules of ATP is used to activate an amino acid, yet only one molecule of ATP is used. Explainarrow_forwardThe molar absorption coefficient of cytochrome P450. an enzyme involved in the breakdown of harmful substances in the liver and small intestine. at 522 nm is 291 dm3 mol-1 cm-1. When light of that wavelength passes through a cell of length 6.5 mm containing a solution of the solute. 39.8 percent of the light was absorbed. What is the molar concentrat ion of the solute?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON
Human Anatomy & Physiology (11th Edition)BiologyISBN:9780134580999Author:Elaine N. Marieb, Katja N. HoehnPublisher:PEARSON Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education,
Anatomy & PhysiologyBiologyISBN:9781259398629Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa StouterPublisher:Mcgraw Hill Education, Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company
Molecular Biology of the Cell (Sixth Edition)BiologyISBN:9780815344322Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter WalterPublisher:W. W. Norton & Company Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co.
Laboratory Manual For Human Anatomy & PhysiologyBiologyISBN:9781260159363Author:Martin, Terry R., Prentice-craver, CynthiaPublisher:McGraw-Hill Publishing Co. Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education
Inquiry Into Life (16th Edition)BiologyISBN:9781260231700Author:Sylvia S. Mader, Michael WindelspechtPublisher:McGraw Hill Education

Human Anatomy & Physiology (11th Edition)
Biology
ISBN:9780134580999
Author:Elaine N. Marieb, Katja N. Hoehn
Publisher:PEARSON

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Anatomy & Physiology
Biology
ISBN:9781259398629
Author:McKinley, Michael P., O'loughlin, Valerie Dean, Bidle, Theresa Stouter
Publisher:Mcgraw Hill Education,

Molecular Biology of the Cell (Sixth Edition)
Biology
ISBN:9780815344322
Author:Bruce Alberts, Alexander D. Johnson, Julian Lewis, David Morgan, Martin Raff, Keith Roberts, Peter Walter
Publisher:W. W. Norton & Company

Laboratory Manual For Human Anatomy & Physiology
Biology
ISBN:9781260159363
Author:Martin, Terry R., Prentice-craver, Cynthia
Publisher:McGraw-Hill Publishing Co.

Inquiry Into Life (16th Edition)
Biology
ISBN:9781260231700
Author:Sylvia S. Mader, Michael Windelspecht
Publisher:McGraw Hill Education
Introduction to the NIOSH Manual of Analytical Methods Fifth edition; Author: Centers for Disease Control and Prevention (CDC);https://www.youtube.com/watch?v=B5rUrKLMoas;License: Standard Youtube License