
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12.10, Problem 12.5P
The infrared spectra for three compounds are provided. Each compound has one or more of the following
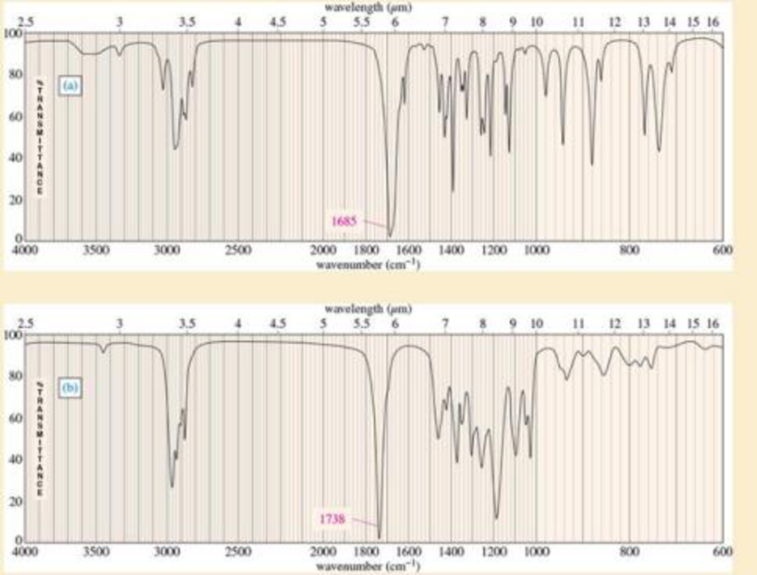
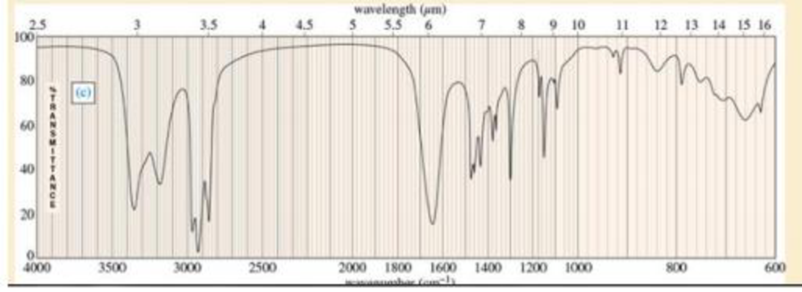
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The naphthalene molecule has a structure that corre-
sponds to two benzene molecules fused together:
The ™ electrons in this molecule are delocalized over the
entire molecule. The wavelength of maximum absorption
in benzene is 255 nm. Will the corresponding wavelength
in naphthalene be shorter or longer than 255 nm?
The infrared spectrum of a compound is unique to the types and number of bonds in the molecule. Draw the structures of salicylic acid and aspirin. What are the key differences of the IR spectrum of aspirin with that of the starting material, salicylic acid? What are the similarities? Interpret the IR spectra of each by assigning the major peaks.
What are 2-3 organic compounds that each contain at least one of the following: carboxylic acid, ester, amine, or amide. How it is synthesized and used, and how the function of the compound is facilitated by its structure and, specifically, its functional groups.
Chapter 12 Solutions
Organic Chemistry (9th Edition)
Ch. 12.3 - Complete the following conversion table. (cm1)...Ch. 12.5 - Which of the bonds shown in red are expected to...Ch. 12.7C - For each hydrocarbon spectrum, determine whether...Ch. 12.9A - Spectra are given for three compounds. Each...Ch. 12.10 - The infrared spectra for three compounds are...Ch. 12.12 - Prob. 12.6PCh. 12.14B - Identify which of these four mass spectra indicate...Ch. 12.15A - Show the fragmentation that accounts for the...Ch. 12.15A - Show the fragmentations that give rise to the...Ch. 12.15B - Ethers are not easily differentiated by their...
Ch. 12.15C - Prob. 12.11PCh. 12 - Prob. 12.12SPCh. 12 - Prob. 12.13SPCh. 12 - All of the following compounds absorb infrared...Ch. 12 - Prob. 12.15SPCh. 12 - Four infrared spectra are shown, corresponding to...Ch. 12 - Predict the masses and the structures of the most...Ch. 12 - Prob. 12.18SPCh. 12 - Prob. 12.19SPCh. 12 - (A true story) While organizing the undergraduate...Ch. 12 - Prob. 12.21SPCh. 12 - Prob. 12.22SPCh. 12 - An unknown, foul-smelling hydrocarbon gives the...Ch. 12 - covered a synthesis of alkynes by a double...Ch. 12 - Three IR spectra are shown, corresponding to three...Ch. 12 - Prob. 12.26SPCh. 12 - Prob. 12.27SPCh. 12 - Prob. 12.28SPCh. 12 - The ultimate test of fluency in MS and IR is...Ch. 12 - Prob. 12.30SPCh. 12 - Consider the following four structures, followed...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How to draw the line bond formula or lewis structure of a methyl ketone with the chemical formula C6H5C3H5O? Based on the results of the solubility tests, the compound can be grouped in VI, which is insoluble in water, 10% NaOH and 10% HCl but soluble in concentrated H2SO4. If the compound is water-insoluble but soluble in concentrated H2SO4, it is most probably an alcohol, aldehyde, anhydride, ester, ether, ketone, or an unsaturated hydrocarbon The functional group/class is identified to be Methyl Ketone, base on the results of the chemical tests on Table 2. CHEMICAL TEST OBSERVATIONS +(compound tested positive for the chemical reaction)/ otherwise (-) Molisch test turbid colorless solution - 2,4-DNP test formation of orange-yellow precipitates + Tollen’s test turbid colorless solution - Ninhydrin test clear pale-yellow solution - iodoform test clear pale-yellow solution +arrow_forwardDescribe two significant differences between the infrared spectra of ethyl alcohol(CH3CH2OH) and ethylene (CH=CH).arrow_forward2. The full structural formulae of three organic compounds, P, Q and R, are shown below. H H H H HH HHHH Н-С -с- Н H-C- C - C = C – H H - C - C = C – C – H H H H H Q P (a) State one similarity between P, Q and R in terms of their molecular formulae. (b) Name the homologous series that compounds P, Q and R belong to. (c) State one similarity between Q and R in terms of chemical bonding id) Which of these compounds are isomers? Explain your answer.arrow_forward
- Estimate the heat released when 1-butene(CH3CH2CHCH2) reacts with bromine to give CH3CH2CHBrCH2Br. Bond enthalpies are CH : 412 kJ/mol; CC : 348 kJ/mol;CC : 612 kJ/mol; CBr : 276 kJ/mol;BrBr : 193 kJ/mol. 1.317 kJ/mol 2.507 kJ/mol 3.95 kJ/mol 4.288 kJ/mol 5.181 kJ/molarrow_forwardView and analyze the given spectroscopic data of an unknown compound with molecular formula C8H10O. Propose a structure based on your analysis. Support your answer by interpreting the given spectroscopic data.arrow_forwardIdentifying organic functional groups Name the family to which each organiC compound belongs. The first answer has been filled in for you. compound family ester CH,– CH,– -0–C – CH, CH; CH, — о — с —Н CH, NH, – C=O CH, Check Explanation O2021 McGraw Hill LLC. All Rights Reserved. Terms ||arrow_forward
- Using GC-MS, as well as NMR and IR spectrometers identify all the compounds. The compounds are likely to be from one of the following classes of compounds: Alcohols and phenols, aldehydes and ketones, amines, or carboxylic acids, amides, and esters.arrow_forwardA C¬D (carbon–deuterium) bond is electronically much like a C¬H bond, and it has a similar stiffness, measured by the spring constant, k. The deuterium atom has twice the mass (m) of a hydrogen atom, however. A chemist dissolves a sample in deuterochloroform (CDCl3) and then decides to take the IR spectrum and simply evaporates most of the CDCl3. What functional group will appear to be present in this IR spectrum as a result of theCDCl3 impurity?arrow_forwardIn the compound below, identify the functional group in the box. A Functional Group in a Compound A hydroxyl B ketone с aldehyde D amine Br O || C Harrow_forward
- Photochemical chlorination of 2,2,4-trimethylpentane gives four isomeric monochlorides. (a) Write structural formulas for these four isomers. (b) The two primary chlorides make up 65% of the monochloride fraction. Assuming that all the primary hydrogens in 2,2,4-trimethylpentane are equally reactive, estimate the percentage of each of the two primary chlorides in the product mixture.arrow_forwardPart 1: Figures 1.1-1.11 belong to some of the organic solvents you will be using this semester. Please write down the STRUCTURE of the compound in the box: ¹H-NMR ¹H-NMR ¹H-NMR EME ¹H-NMR 3 Figure 1.1: The structure of this Tertiary amine (molecular formula C6H15N) is: Figure 1.2: The structure of the Secondary amine is (molecular weight 73): Figure 1.3: The structure of this primary alcohol (C4H10O) is: Figure 1.4: The structure of this ester (C4H8O2) is: Page 1 of 4arrow_forwardWrite the systematic name of each organic molecule: structure OH H-C-CH-CH-CH₂-OH I CH3 CH3 I CH,—CH–CH2−CH O || H—C–CH–CH3 I CH3 name 0 0 Xarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
NMR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=SBir5wUS3Bo;License: Standard YouTube License, CC-BY