
Concept explainers
In the following compound, identify whether each lone pair is available to function as a base, and explain your choice:
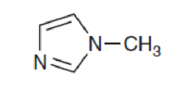
Interpretation:
The availability of lone pair in the compound to function as a base has to be explained.
Concept Introduction:
If a lone pair is not a part of the ring means not taking part in resonance, then it will be available to function as a base. It also can be explained in other way. Like if a lone pair is present in a
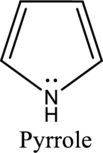
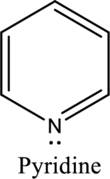
Nitrogen in both pyrrole and pyridine has a lone pair and it is
Explanation of Solution
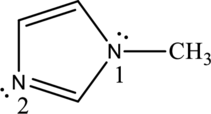
By properly examining the structure, it can be seen that the numbered
The numbered
Want to see more full solutions like this?
Chapter 1 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Additional Science Textbook Solutions
Inorganic Chemistry
General, Organic, and Biological Chemistry (3rd Edition)
Living by Chemistry
Living By Chemistry: First Edition Textbook
Elementary Principles of Chemical Processes, Binder Ready Version
- Rank the following compounds in terms of increasing acidity (least acidic first). Explain your ranking. Making sure to say which hydrogen in each molecule is the most acidic, and discuss the relative stability of the conjugate bases.arrow_forwardOne of the three molecules is much more acidic than the other two. Identify the molecule and explain why it is so much more acidic that the other two. H Harrow_forwardWhich of the following anions is the stronger base? Explain your choice by typing your answer below. A Вarrow_forward
- Which of the following anions is the stronger base? Explain your choice.arrow_forwardArrange the following compounds in order of increasing acidity by typing in the numbers underneath them. For example, an answer of 1234 would indicate that you think 1 is least acidic and 4 is most acidic (with 4 being more acidic than 3 and so forth). You should only consider the most acidic proton on each molecule. CF3 НО 2 HO 4 1 3 Enter Your Answer:arrow_forwardThe antimalaria properties of quinine (C20H24N202) saved thousands of lives during construction of the Panama Canal. This substance is a classic example of the medicinal wealth that tropical forests hold. Both N atoms are basic, and the N of the amine group is far more basic (pKb=4.94) than the N within the aromatic ring system (pKb=10.54). A saturated solution of quinine in water is only 4.55x10-3M. What is the pH of this solution? Record your answer with 2 decimals.arrow_forward
- The ammonium ion (pKa = 9.25) has a lower pKa than the methyl ammonium ion (pKa = 10.66). Explain which of the two species is the stronger basearrow_forwardcomplete the equation for the reaction between each Lewis acid-base pair. In each equation, label which starting material is the Lewis acid and which is the Lewis base; use curved arrows to show the flow of electrons in each reaction. In doing this problem, it is essential that you show valence electrons for all atoms participating in each reactionarrow_forwardExplain why phenol is more acidic than alcohols by considering the resonance effect anddrawing the resonance structures of phenoxide ions.arrow_forward
- Trifluoroethanol (CF3CH2OH, pKa = 12.5) is more acidic than ethanol (CH3CH2OH, pKa = 16). Provide a three-dimensional structure for each conjugate base and draw the orbitals for all lone pairs. Provide a succinct explanation for the greater acidity of trifluoroethanol.arrow_forwardThe following two compounds are constitutional isomers. Identify which of these is expected to be more acidic, and explain your choice. OH مل H₂C OH A is the stronger acid because the negative charge in the conjugate base is adjacent to an electron-withdrawing group, making it the LESS stable conjugate base. O A is the stronger acid because the negative charge in the conjugate base is adjacent to an electron-withdrawing group, making it the MORE stable conjugate base. O B is the stronger acid because the negative charge in stable conjugate base. the conjugate base is delocalized by resonance, making it the MORE B is the stronger acid because the negative charge in the conjugate base is delocalized by resonance, making it the LESS stable conjugate base.arrow_forward3, Which of the molecules drawn below is most acidic? Draw the conjugate base and explain thoroughly. वे देई d a C barrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
