
Organic Chemistry (9th Edition)
9th Edition
ISBN: 9780321971371
Author: Leroy G. Wade, Jan W. Simek
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 15.13D, Problem 15.22P
Using the examples in Table15-2 to guide you, match four of the following UV absorption maxima (λmax) with the corresponding compounds: (1) 232 nm; (2) 256 nm; (3) 273 nm; (4) 292 nm; (5) 313 nm; (6) 353 nm.
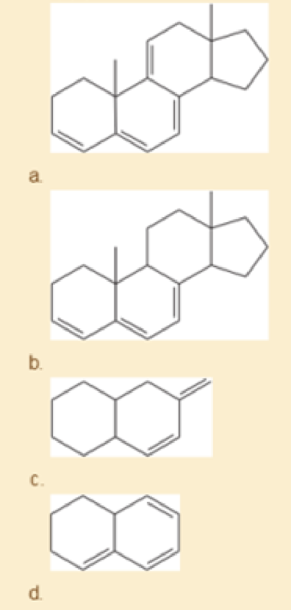
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
. Deduce the structure of an unknown compound with the molecular formula of
C5H₁2O using the information given by its infrared spectrum. ANSWER
A)
НО.
B)
D)
Intensity Frequency
(peak): (cm):
3300
m
EEEEE
m
m
m
m
m
2900
2800
1465
1450
1375
The 1H NMR spectrum, 13C NMR spectrum, mass spectrum, and IR spectrum below belong to a chemical with the molecular formula C4H9XO, where X is a halogen. Provide a structure for that compound. You must explain how you determined the structure for full credit based on the data bellow.
The IR spectra of nonane (C,H20) and 1-hexanol
(C,H13OH) are shown below. Assign each spectrum to the
correct compound and identify the frequencies and the
functional groups used to support your assignment.
λ (μη)
6 7 8 9 10 11 12 1314 15
2.5
100
3
5
20
4
90
80
70
60
50
40
30
20
10
4000 3600 3200 2800 2400
2000 1800 1600 1400 1200 1000
800
600
400
Frequency (cm¬')
A (µm)
2.5
100
8 9 10 11 12 13 14 15
3
4
5
7
20
90
80
70
60
50
40
30
20
10
4000 3600 3200 2800 2400
2000 1800 1600 1400 1200 1000
800
600
400
Frequency (cm¬!)
(%) Transmission
(%) Transmission
Chapter 15 Solutions
Organic Chemistry (9th Edition)
Ch. 15.2 - Prob. 15.1PCh. 15.2 - Prob. 15.2PCh. 15.2 - Prob. 15.3PCh. 15.4 - Prob. 15.4PCh. 15.4 - Prob. 15.5PCh. 15.5 - Treatment of an alkyl halide with AgNO3 in alcohol...Ch. 15.5 - Propose a mechanism for each reaction, showing...Ch. 15.6 - When Br2 is added to buta-1,3-diene at 15 C, the...Ch. 15.7 - Prob. 15.9PCh. 15.7 - When N-bromosuccinimide is added to hex-1-ene in...
Ch. 15.7 - Prob. 15.11PCh. 15.9 - Addition of 1-bromobut-2-ene to magnesium metal in...Ch. 15.10 - Show how you might synthesize the following...Ch. 15.11 - Predict the products of the following proposed...Ch. 15.11 - Prob. 15.15PCh. 15.11A - Prob. 15.16PCh. 15.11B - Prob. 15.17PCh. 15.11B - Predict the products of the following Diels-Alder...Ch. 15.12C - Prob. 15.19PCh. 15.12C - Prob. 15.20PCh. 15.13C - Prob. 15.21PCh. 15.13D - Using the examples in Table15-2 to guide you,...Ch. 15.14 - Phenolphthalein is an acid-base indicator that is...Ch. 15 - Prob. 15.24SPCh. 15 - Prob. 15.25SPCh. 15 - Show how the reaction of an allylic halide with a...Ch. 15 - Prob. 15.27SPCh. 15 - A solution was prepared using 0.0010 g of an...Ch. 15 - Prob. 15.29SPCh. 15 - Prob. 15.30SPCh. 15 - Prob. 15.31SPCh. 15 - Prob. 15.32SPCh. 15 - Prob. 15.33SPCh. 15 - Give the structures of the products represented by...Ch. 15 - Furan and malemide undergo a Diels-Alder reaction...Ch. 15 - Prob. 15.36SPCh. 15 - Prob. 15.37SPCh. 15 - Prob. 15.38SPCh. 15 - Prob. 15.39SPCh. 15 - Determine whether each structure is likely to be...Ch. 15 - An important variation of the Diels-Alder reaction...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Calculate the IHD of C7H6XNO and identify the important peaks in the following MS spectral data and draw the structure of the important peaks in the following MS spectral data.arrow_forward(d) Indicate for three of the following pairs of compounds which spectroscopic method you would choose to distinguish between them [there may be more than one 'correct' technique]. Explain your choice and outline the differences you would expect to observe. OH DOH OH O OMe L MeO OMe, OH OH Pair D Pair E Pair F Pair G HO OMe ว MeO OMe, OH OHarrow_forward1B. Can 1H NMR spectroscopy be used to differentiate between the two compounds? Briefly explain why or why not. Predict the 1H NMR spectrum for each compound (include integration, multiplicity, and approximate chemical shift). Put it in data table format.arrow_forward
- Identify and draw the structure of the important peaks in the MS spectral data of C9H8O given below.arrow_forward5. Which of the following compounds would you expect to show ultraviolet absorptions in the 200 to 400 nm range? (a) (b) (c) CN (d) (e) CH3 (f) HO. H.arrow_forward- (a) Compounds A and B having identical molecular formula C4H80 have the following C signals at d= 7 ·87, 29.43, 36. 87, 209· 28 ppm and 8 = 13·71, 15.69, 45· 85 and 202·80, respectively. DEPT gave a single-inverted peak for A and two inverted peaks for B. Predict the two structures of A and B, assign peaks and identify the functional groups. 13 %3Darrow_forward
- A compound (L) with the molecular formula C9H10 reacts with bromine and gives an IR absorption spectrum that includes the following absorption peaks: 3035 cm ¹(m), 3020 cm ¹(m), 2925 cm ¹(m), 2853 cm ¹(w), 1640 cm ¹1(m), 990 cm ¹(s), 915 cm ¹(s), 740 cm ¹(s), 695 cm ¹(s). The ¹H NMR spectrum of L consists of: Doublet 3.1 ppm (2H) Multiplet 5.1 ppm Multiplet 7.1 (5H) ppm Multiplet 4.8 ppm Multiplet 5.8 ppm The UV spectrum shows a maximum at 255 nm. Propose a structure for compound L. Edit Drawing harrow_forwardThe following molecular ion isotopic clusters correspond to derivatives of benzene (C6H6) for which one or more of the hydrogens have been replaced with chlorines or bromines. What molecular formula corresponds to each mass spectrum? . 100- 80- 60- 40- Relative intensity 20- -M+6 -M+5 -M+4 M+3 M+1 M+2 M+0 (a) -M+6 -M+5 -M+4 M+3 M+2 M+1 -M+0 (b) M+6 M+5 M+4 M+3 -M+2 -M+1 -M+0 (c) M+6 -M+5 M+4 M+3 M+2 -M+1 -M+0 (d) M+6 M+5 -M+4 -M+3 -M+2 M+1 -M+0 (e)arrow_forwardIdentify the two geometric isomers of stilbene, C6H5CH=CHC6H5 from their λmax values, 294 nm and 278 nmarrow_forward
- The chemical formula is C8H12O. What is it’s structure and degrees of unsaturation? What important functional groups are in the spectra, and what peaks correspond with which hydrogens and carbons in the structure predicted.arrow_forwardWhat functional group and molecule can you get from its IR spectrum? Support your answer by identifying prominent peaks (wavenumbers cm^-1) and assigning the functional group.arrow_forward3A2: Integrate their knowledge of 2, symmetry, and molecular formula (MF) with ¹3C NMR data and determine organic structure. Write a line-angle structure that is consistent with a MF of C7H10O and the 13C NMR data provided. You must label all the carbon atoms in your structure with the correct Roman letters (a, b, c, d, etc.) in the NMR data. ¹H Decoupled a b c b c ULILJAJ 0 240 ppm 240 ppm DEPT 135° d ef d DEPT 90° 0 240 b c ppm 0arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
IR Spectroscopy; Author: Professor Dave Explains;https://www.youtube.com/watch?v=_TmevMf-Zgs;License: Standard YouTube License, CC-BY