
Concept explainers
Assign the peaks in the
NMR spectrum of eugenol (Fig. 6.23) to specific protons in the molecule. The OH peak is at 5.1 ppm.
Interpretation:
The peak in
Concept introduction:
NMR is an analytical method that is utilized to identify the structure of a compound. It determines the framework of carbon-hydrogen bonds present in the compound.
Number of signals in the
The protons that are present in the different chemical environment are known as non-equivalent protons while the protons that are present in same chemical environment are known as equivalent protons.
Answer to Problem 1Q
Eugenol has 12 different hydrogen atoms. The three hydrogen atom present on the aromatic ring show peak around
Explanation of Solution
The structure of eugenol is as follows:
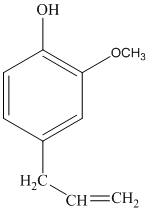
Eugenol has 12 different hydrogen atoms. The integration of peaks from right to left is 3:2:2:3:2.
There are two peaks for the three hydrogens around
There are two peaks for four hydrogen atoms around
There are two peaks for six around
Want to see more full solutions like this?
Chapter 6 Solutions
Macroscale and Microscale Organic Experiments
- Label the signals due to Ha, Hb, and Hc in the 1H NMR spectrum of acrylonitrile (CH2=CHCN). Draw a splitting diagram for the absorption due to the Ha proton.arrow_forwardFor the 1 H NMR spectrum of acetyl salicylic acid shown below, assign each signal to the proton to which it corresponds (the COOH proton does not appear on this spectrum).arrow_forwardWhich compounds give a 1H NMR spectrum with two signals in a ratio of 2:3?arrow_forward
- Describe the 1H NMR spectrum of each compound. State how many NMR signals are present, the splitting pattern for each signal, and the approximate chemical shift.arrow_forwardA'2 The 'H-NMR spectra of cyclohexanol and cyclohexanone are given below. Identify which spectrum belongs to which compound and assign the peaks in each spectrum that substantiate your decision.arrow_forwardWhat is the structure of an unknown compound with molecular formula C6H15N that gives the following 1H NMR absorptions: 0.9 (singlet, 1 H), 1.10 (triplet, 3 H), 1.15 (singlet, 9 H), and 2.6 (quartet, 2 H) ppm?arrow_forward
- 1Compound 1 has molecular formula C7H16. It shows three signals in the 1H-NMR spectrum, one at 0.85 ppm, one at 1.02 ppm, and one at 1.62 ppm. The relative integrals of these three signals are 6, 1, and 1, respectively. Compound 2 has molecular formula C7H14. It shows three signals in the 1H-NMR spectrum, one at 0.98 ppm, one at 1.36 ppm, and one at 1.55 ppm. The relative integrals of these three signals are 3, 2, and 2, respectively. Propose structures for compounds 1 and 2, explaining how you reach your conclusion.arrow_forwardShown below is the H-NMR spectra of phenacetin. Draw the structure and correlate each hydrogen in the molecule with the various peaks in the spectra.arrow_forwardDraw the structure of C3H8O and indicate which sets of hydrogens correspond to which signal in the H NMR spectrum.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





