
Concept explainers
- a. Draw the resonance forms for SO2 (bonded O—S—O).
- b. Draw the resonance forms for ozone (bonded O—O—O).
- c. Sulfur dioxide has one more resonance form than ozone Explain why this structure is not possible for ozone.
(a)
To determine: The resonating structures of
Interpretation: The resonating structures of
Concept introduction: Resonance is the process in which a molecule gets different structures to define its bonding within the molecule. Such molecules cannot be represented in single Lewis structures. Resonating structures of such molecules are called contributing structures. In the process of resonance, shifting of lone pairs occurs with the bonds and other lone pairs.
Answer to Problem 1.26SP
The resonating structures of
Explanation of Solution
The resonating structures of the given molecule are shown as,
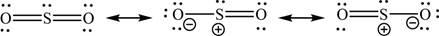
Figure 1
The given compound is
(b)
To determine: The resonating structures of
Interpretation: The resonating structures of
Concept introduction: Resonance is the process in which a molecule gets different structures to define its bonding within the molecule. Such molecules cannot be represented in single Lewis structures. Resonating structures of such molecules are called contributing structures. In the process of resonance, shifting of lone pairs occurs with the bonds and other lone pairs.
Answer to Problem 1.26SP
The resonating structures of the given molecule are shown in Figure 2.
Explanation of Solution
The resonating structures of the given molecule are shown as,

Figure 2
The given compound is trioxygen. In this molecule, three oxygen atoms are joined together by one double and one single bond. One oxygen atom carries a positive charge and one carries a negative charge. The positive charge, negative charge and a double bond present in conjugation in the given molecule. It results in the resonating structures of
(c)
To determine: The reason corresponding to the fact that ozone has only two resonating structures, whereas sulfur dioxide has three.
Interpretation: The reason corresponding to the given fact is to be stated.
Concept introduction: Resonance is the process in which a molecule gets different structures to define its bonding within the molecule. Such molecules cannot be represented in single Lewis structures. Resonating structures of such molecules are called contributing structures. In the process of resonance, shifting of lone pairs occurs with the bonds and other lone pairs.
Answer to Problem 1.26SP
The reason corresponding to the given fact is stated below.
Explanation of Solution
Both the sulfur dioxide and ozone molecules are triatomic and both contain atoms of oxygen family. But the resonating structures of sulfur dioxide are more than ozone. The main reason for this is the difference in the group number of the central atom.
In sulfur dioxide, sulfur is present as a central atom, whereas in ozone oxygen atom is present as a central atom. Rest of the surrounding atoms in both the molecules are same. Atomic number of sulfur is sixteen. Whereas, oxygen has the atomic number eight. Therefore, sulfur contains third shell too and it is capable to handle extra bond by using empty “d” orbital and also able to show more resonating structures. This is not the case with ozone molecule. Hence, sulfur dioxide has more resonating structures than ozone molecule.
Want to see more full solutions like this?
Chapter 1 Solutions
Organic Chemistry (9th Edition)
Additional Science Textbook Solutions
Introductory Chemistry (5th Edition) (Standalone Book)
General Chemistry: Atoms First
Chemistry: Structure and Properties (2nd Edition)
Chemistry: A Molecular Approach (4th Edition)
Organic Chemistry
Essential Organic Chemistry (3rd Edition)
- Draw the best Lewis structure for SiO3?- How many total valence electrons do you have available? Which atom goes in the center? Draw the structure with single bonds only. Is this the final structure? Why or why not?arrow_forwardb. There is one additional resonance structure. NH₂ c. There is a total of five resonance structures (including the original structure). : OHarrow_forwardDraw the Lewis structure for CH4O. b. Draw the Lewis structure for HNO2arrow_forward
- B. Write the Lewis Structure for SO2. Include all resonance structures. C. Write the Lewis Structure for SF4. Then predict the molecular shape, give the bond angle(s), give the expected hybridization, and give the polarity of the molecule. Include all answers in the box.arrow_forwardLewis structure rules don't always work. In some cases, an atom can be stable with fewer or more than 8 electrons in the valence shell. In some cases, the sum of all valence electrons in the molecule adds up to an odd number and you will have an unpaired electron. a. Explain why nitrogen cannot have an expanded octet but phosphorus can. b. Draw the Lewis structure for the following molecules. i. BeF2 ii. SF4 ii. ICIarrow_forward2. The Lewis structure for the acetate ion [C₂H3O2]¹ is given below: le a. Read section 1.9. What is the geometry (shape) around each of the carbon atoms in the acetate ion - tetrahedral, trigonal planar or linear? b. Each of the oxygen atoms in the acetate ion have an octet. Draw all lone pairs of electrons in the acetate ion given above. c. Read section 1.7. Use electron pushing to generate a resonance structure of the acetate Lewis structure given above.arrow_forward
- The formula for nitryl chloride is CINO2 (in which N is the central atom). a.Draw the Lewis structure for the molecule, including all resonance structures. b.What is the N-O bond order? c.Describe the electron-pair and molecular geometries and give values for all bond angles. d.What is the most polar bond in the molecule? Is the molecule polar? e.The computer program used to calculate electrostatic potential surfaces gave the following charges on atoms in the molecule: A =-0.03, B = -0.26, and C = +0.56. Identify the atoms A, B, and C. Are these calculated charges in accord with your predictions?arrow_forward2. Avogadro does not "waste" his time drawing a Lewis structure before determining the shape of PF3. He thinks that the shape of PF3 must be trigonal planar because there are three fluorine atoms bonded to the central phosphorus atom. a. Draw the Lewis structure for PF3. b. Was Avogadro's answer for the shape of a PF3 molecule correct? Explain c. Why is it important to draw the Lewis structure for a molecule before identifying the shape of the molecule?arrow_forward2. Avogadro does not "waste" his time drawing a Lewis structure before determining the shape of PF3. He thinks that the shape of PF3 must be trigonal planar because there are three fluorine atoms bonded to the central phosphorus atom. a. Draw the Lewis structure for PF3. b. Was Avogadro's answer for the shape of a PF3 molecule correct? Explain c. Why is it important to draw the Lewis structure for a molecule before identifying the shape of the molecule? 3. Draw the Lewis structure of ozone, O3. Describe why ozone has a bent shape instead of a linear shape.arrow_forward
- Draw the Lewis structure for NO2, including any valid resonance structures. Which of the following statements is TRUE? O a. The nitrite ion contains two N-O single bonds. O b. The nitrite ion contains two N=O double bonds. Oc. The nitrite ion contains two N-O bonds that are equivalent to 1 bonds. O d. The nitrite ion contains one N-O single bond and one N=O double bond. O e. None of the other choices is correctarrow_forwardFill in the table. Central atom is listed first. A. Write the number of valence electrons below the formulaB. Draw the Lewis structureC & D. Write the Electron Group Geometry and Molecular Shape NamesE. Write the bond angleF. Write the molecular polarity. "P" for polar and "NP" for nonpolar. SpeciesValenceElectrons(1 pt.) LewisStructure(2 pt.) Electron PairGeometryName(1 pt.) Molecular ShapeName (1 pts.) BondAngle (1 pt.) Molecular Polarity(1 pt.) PO43- NOBr Uploadarrow_forwardIdentify if the statement is true or false. If false, explain why the statement is false. a. H for the formation of a bond is always a negative number. b. Because the presence of pi bonds does not influence the geometry of a molecule, the presence of pi bonds does not affect the value of the bond enthalpy between two atoms. c. A hot metal is placed in a calorimeter. The temperature of the water increases. The q value for the metal will be positive and the q value for the water will be negative.arrow_forward
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning




