
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
4th Edition
ISBN: 9781119776741
Author: Klein
Publisher: WILEY CONS
expand_more
expand_more
format_list_bulleted
Question
Chapter 10.3, Problem 4LTS
Interpretation Introduction
Interpretation:
A mechanism for the radical chlorination of methyl chloride to produce methylene chloride needs to be drawn:
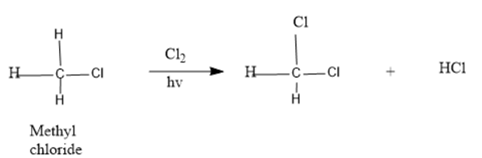
Concept Introduction :
Free-radical halogenation is a form of halogenation in organic chemistry.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Complete the following reactions and outline the mechanism for the formation of the product:
5c) Write the rate equation for the following SN1 reaction:
what will be the type of interaction of ethane with bromine:
a) radicals
b) ionic
c) nucleophilic
d) electrophilic
Chapter 10 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
Knowledge Booster
Similar questions
- Alkyl halides can be reduced to alkanes by a radical reaction with tributyltin hydride, (C4H9)3SnH, in the presence of light (hv). Propose a radical chain mechanism by which the reaction might occur. The initiation step is the light-induced homolytic cleavage of the Sn-H bond to yield a tributyltin radical.arrow_forwardIn the presence of a radical initiator (Z•), tributyltin hydride (R3SnH, R = CH3CH2CH2CH2) reduces alkyl halides to alkanes: R′X + R3SnH → R′H + R3SnX. The mechanism consists of a radical chain process with an intermediate tin radical: This reaction has been employed in many radical cyclization reactions. Draw a stepwise mechanism for the following reaction.arrow_forwardReaction of but-1-ene with HBr gives two products in unequal amounts. In each case, identify the two products, state which is the major product, explain why it is the major product and give the mechanism for its formation.arrow_forward
- Peroxides are often added to free-radical reactions as initiators because the oxygen–oxygen bond cleaves homolytically rather easily. For example, the bond-dissociation enthalpy of the O¬O bond in hydrogen peroxide (H¬O¬O¬H) is only 213 kJ>mol (51 kcal>mol). Give a mechanism for the hydrogen peroxide-initiated reaction of cyclopentane with chlorine. The BDE for HO¬Cl is 210 kJ>mol (50 kcal>mol).arrow_forwardConsider the reaction of NaCl with 2- bromo-2-methylhexane in acetone/water. Predict the change in rate if the concentrations of both the 2-bromo-2- methylhexane and the NaCl are doubled. A) The reaction rate stays the same. B) The reaction rate doubles. C) The reaction rate is halved. D) The reaction rate quadruples E) The reaction rate triples.arrow_forwardA problem often encountered in the oxidation of primary alcohols to acids is that esters are sometimes produced as by-products. For example, oxidation of ethanol yields acetic acid and ethyl acetate: Propose a mechanism to account for the formation of ethyl acetate. Take into account the reversible reaction between aldehydes and alcohols:arrow_forward
- The mechanism that allows the exothermic hydrolysis of t-butyl chloride to form t-butyl alcohol and chloride ion is as follows: (CH3)3CCl → (CH3)3C+ + Cl− (CH3)3C+ + H2O → (CH3)3COH + H+ Draw the transition state for each step.arrow_forwardProvide the whole reaction mechanism (generation of electrophile, nucleophile, bond formation, bond breaking and movement of arrows) and the final product of the following reactions:arrow_forwardThe reaction of methylpropene with HBr, under radical conditions, gives two intermediates. Propose a mechanism for the formation of the two products. Propose a mechanism for the following reaction and use electronic factors to account for the formation of a major product: CH2 CH2Br N-Bromosuccinimide (NBS) ho, CCI4 Draw the structure of an antioxidant, Vitamin E free radical and use resonance structures o account for its stability.arrow_forward
- In the synthesis of 1,2-dibromopropane from propan-1-ol: how many steps does it take in the shortest route?arrow_forwardPara-substituted product was produced when phenol reacts with cyclohexanecarbonyl bromide in the presence of AIB13. -Br Cyclohexanecarbonyl bromide (i) Outline the mechanism for this reaction. (ii) Draw the alternative substituted product formed.arrow_forward2-chloro-1,1,3-trimethylcyclohexane can undergo either substitution or elimination or BOTH reactions. Determine ALL possible type of reaction occur (E1, E2, SN1 or SN2), propose a detailed mechanism (or mechanisms) for reaction and identity the MAJOR compounds when; the reagent used is butanol he reagent used is butoxide the reagent used is (CH),0arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you


 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning



Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning