
Concept explainers
(a)
Interpretation:
The product formed in the reaction of 1-Hexanol with chromic acid has to be drawn.
Concept Introduction:
Oxidation of alcohols: Oxidation of primary alcohol gives an
Chromic acid Oxidation of an Alcohol:
Step 1: Reaction of alcohol and chromic acid gives an alkyl chromate. No change in oxidation state of both Carbon and Chromium.
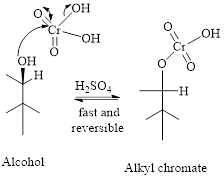
Step 2: Take a proton away and simultaneously break bonds to give stable molecules or ions. Reaction of the alkyl chromate with a base (here water molecule) results in cleavage of a
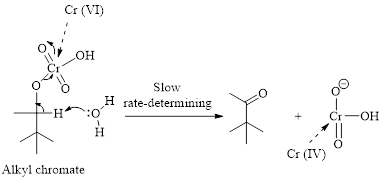
(b)
Interpretation:
The product formed in the reaction of 2-Hexanol with chromic acid has to be drawn.
Concept Introduction:
Oxidation of alcohols: Oxidation of primary alcohol gives an aldehyde or a carboxylic acid, depending on experimental conditions.
Chromic acid Oxidation of an Alcohol:
Step 1: Reaction of alcohol and chromic acid gives an alkyl chromate. No change in oxidation state of both Carbon and Chromium.

Step 2: Take a proton away and simultaneously break bonds to give stable molecules or ions. Reaction of the alkyl chromate with a base (here water molecule) results in cleavage of a

(c)
Interpretation:
The product formed in the reaction of Cyclohexanol with chromic acid has to be drawn.
Concept Introduction:
Oxidation of alcohols: Oxidation of primary alcohol gives an aldehyde or a carboxylic acid, depending on experimental conditions.
Chromic acid Oxidation of an Alcohol:
Step 1: Reaction of alcohol and chromic acid gives an alkyl chromate. No change in oxidation state of both Carbon and Chromium.

Step 2: Take a proton away and simultaneously break bonds to give stable molecules or ions. Reaction of the alkyl chromate with a base (here water molecule) results in cleavage of a

Trending nowThis is a popular solution!

Chapter 10 Solutions
Organic Chemistry
- (a) Draw two different enol tautomers of 2-methylcyclohexanone. (b) Draw two constitutional isomers that are not tautomers, but contain a C=C and an OH group. 2-methylcyclohexanonearrow_forwardWhich of (a)-(d) is the correct IUPAC name of the following compound? * HO O 3-methyl-1-phenyl-1-pentanol ○ 3-methyl-5-phenyl-5-pentanol ○ 3-methyl-1-benzene-1-pentanol O 1-phenyl-1-hexanolarrow_forwardA) Provide four different ways that 2-methylpropanal could be made from an alkene, an alcohol, an ester and an acetal in a single step. an alkene H an alcohol 2-methylpropanal an ester an acetalarrow_forward
- Draw the structures that correspond to their IUPAC or common name. (c) 4-mercapto-1-butanol (d) trans-1,3-diethoxycyclopentanearrow_forward4. Which of the following has a higher boiling point? Explain your answer:(a) 2-propanol or 2-butanol(b) Dimethyl ether or ethanolarrow_forward2. Draw one enol tautomer of each of the following ketones and aldehydes. a) b) c) of مل G Harrow_forward
- For each of the following compounds, draw its structure. a) 2,4,6-trimethyl-3-nonanone b) trans-2-tert-butylcyclohexanol c) sec.-butyl propyl ether gamma-bromo-beta-methoxybutyraldehyde d) e) 3,3-diethyl-2-hexanethiolarrow_forwardProvide the classification (alcohol or ether) and provide the correct IUPAC name of each molecule. CH3 H3C. ОН b) Namena 1a) Classification: CH3 H3C. b) Namena 2a) Classification: CH3 H3C CH3 b) Nameina 3a) Classification: CH3 H3C он b) Namena 4a) Classification: CI H3C. CH3 OH ÓH CI b) Namena 5a) Classification:arrow_forwardc) rite in the reagent(s) over the arrow. a) C6H5N₂+ b) C6H5C=N H3C- An OH H₂C → benzene H3C benzylamine CI CH3arrow_forward
- (a) Draw the structure of the hemiacetal formed from one mole of benzaldehyde and one mole of ethanol. (b) Draw the structure of the acetal formed from one mole of benzaldehyde and two moles of ethanol. (c) Draw the structure of 2-methoxy-2-butanol. What compounds could you prepare this from? (d) Draw the structure of 3-methoxyl-2-butanol. What functional groups are present? Is this an acetal, a hemiacetal, or neither? Explain. (e) Identify the functional groups in the molecules shown below. Circle any acetals or hemiacetal, and identify which they are. 0-arrow_forwardDraw the structure of the major organic product(s) for the following reaction between an acetylenic anion and an alkyl halide. (The reaction stoichiometry is 1:1.)arrow_forwardWe have covered several oxidants that use a multi-valent atom (Cr, Cl, S, or I) as their active species, going from a higher oxidation state before the oxidation to a lower oxidation state after oxidizing the alcohol. Draw the structure of the following atoms, before and after the oxidation of an alcohol to a ketone or aldehyde. How many bonds to oxygen does each atom have before and after the oxidation? (a) the I in the DMP reagent (b) the carbinol C in the alcohol that is oxidizedarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





