
The following experimental data were obtained for the reaction of \'I14* and NOf in acidic solution.
NH/(aq) + NO2-(aq) — N;(g) + 2 H,O(f)
| INH/I (mol L1) | [NO21 (mol L-1, | Rate = A[NJ/At (mol L-1 s’) |
| 0.0092 | 0.098 | 3.33 X IO"7 |
| 0.0092 | 0.049 | 1.66 X 10‘7 |
| 0.0488 | 0.196 | 3.51 X 10"6 |
| 0.0249 | 0.196 | 1.80 X 10-6 |
Determine the rate law for this reaction and calculate the rate constant.
Interpretation: Given the experimental data obtained for a reaction, determine the rate law for the reaction and the value of rate constant
Concept Introduction: Orders of reaction are constantly determined by doing experiments. Consequently without experimental information, we can't conclude anything about the order of a reaction just by having a look at the equation for the reaction. By doing experiments involving a reaction between A and B, the rate of the reaction is identified to be related to the concentrations of A and B as follows:
This is the Rate Equation.
Where,
Rate is in the units of mol dm-3s-1
k is the rate constant
A, B- concentrations in mol dm-3
a - Order of reaction with respect to A
b- Order of reaction with respect to B
Answer to Problem 11.33PAE
Solution: The rate law of the reaction is
Explanation of Solution
Given information: Reaction of
Experimental Data
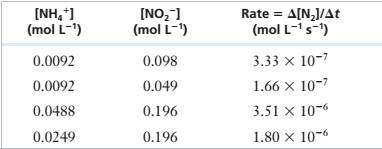
Step 1: For the reaction:
The rate law can be determined using the rate equation as follows:
Where,
a= Order of the reaction with respect to
b= Order of the reaction with respect to
Step 2: From the first and second rows of the given experimental data,
Step 3: Divide (1) by (2), we get
Step 4: From the third and fourth rows of the given experimental data,
Step 5: Divide (3) by (4), we get
Step 6: Rate Equation = >
To determine k, pick equation (1)
It does not make a difference what the number of reactants there are. The concentration of every reactant will be present in the rate equation, raised to some power. These powers resemble the individual orders with respect to each reactant. The sum of these powers results in the overall order of the reaction. The rate constant will be a constant value for a given reaction only if the concentration of the reactants is changed without changing any other factors.
Want to see more full solutions like this?
Chapter 11 Solutions
Chemistry for Engineering Students
- The following data were obtained for the reaction 2C102 (ag) + 20H (ag) → Cl0; (ag) + C102 (ag) + H,0(1) A[CIO2] where Rate = - At [C102]o (mol L) [OH ], (mol L) Initial Rate (molL·s) 0.0750 0.150 1.94 x 10-1 0.150 0.150 7.76 x 10-1 0.150 0.0750 3.88 x 10-1 a Determine the rate law. (Use k for the rate constant.) Rate = k[ClO,][OH] b Determine the value of the rate constant. Rate constant = 2.30x1 / L²/mol? - s c What would be the initial rate for an experiment with [CIO2], = 0.140 mol/L and [OH], = 0.0668 mol/L? Rate = mol/L·sarrow_forwardObtain the 2"-1 – 1 | T (п - 1)k. а"-1 relation between the order of the reaction (n), the reaction rate constant (k), the initial concentration (a), and the half-life (T).arrow_forwardIn a study of the decomposition of hydrogen peroxide in dilute sodium hydroxide at 20 °C HzOz(aq)+HzO(l) + % Oz(E) the concentration of H₂O₂ was followed as a function of time. It was found that a graph of In[H₂O₂] versus time in minutes gave a straight line with a slope of -1.51×10-3 min-1 and a y-intercept of -3.47. min-1. Based on this plot, the reaction is ✓order in H₂O₂ and the rate constant for the reaction isarrow_forward
- 6. To observe the effect of temperature on reaction rate, the reaction between MnO, ag) and C204(ag) in acidic media is performed according to the given redox reaction: 2 MnO4 (aq) + 16 H*(aq) + 5 C204²(aq) → 10 CO2(g) + 2 Mn²"(aq) + 8 H2O(1) At various temperatures, the rate constants were determined experimentally as follows: k (M s') 5.03x105 T (°C) 20 35 3.68×104 60 6.71×103 90 0.119 (a) Determine Ea for this reaction by a graphical method (see the graph given below). (b) Determine E. using Arrhenius equation using tabulated data. (c) Calculate the value of the rate constant, k at 100°C. -2 -4 兰 -6 -8 -10 -12 0.0025 0.0027 0.0029 0.0031 0.0033 0,0035 1/T (K)arrow_forwardIn a study of the conversion of methyl isonitrile to acetonitrile in the gas phase at 250 °CCH3NC(g)CH3CN(g)the concentration of CH3NC was followed as a function of time.It was found that a graph of ln[CH3NC] versus time in seconds gave a straight line with a slope of -4.38×10-3 s-1 and a y-intercept of -2.83 . Based on this plot, the reaction is ______ order in CH3NC and the half life for the reaction is _______ seconds.arrow_forwardExpt. 1 2 3 NH; (aq) + NO; (aq) >Nz (aq) + 2 H2O (l) [NO₂] Rate (at 298 K) 0.020 M/s 0.020 M 0.020 M 0.030 M/s 0.010 M 0.0050 M/s [NH+] 0.010 M 0.015 M 0.010 M Determine the complete rate law (rate law and rate constant) for the reaction. Show all work. What is the initial rate of this reaction if the concentrations in Expt. 2 above are tripled. Show work. What is the rate of H₂O formation in Expt. 1? A 4th trial is conducted at 273 K, using the concentrations from Expt. 1. If the rate was found to be 0.016 M/s, calculate the activation energy of this reaction.arrow_forward
- Consider chemical reaction:- X +2Y+ az )XY2Z2 It is faund that mng the [X] quadaup the [2] oubles the hate -) doubling the Cx7 has no effect on the sate doulbl les the late dneponb Cx] (- what is -the fate seaction 9 2 Pse dict the reactants for the Tate determ inA step. what is the molecularlty of the rate-deter step. minir 2) Predict a Complete mechanism Ahat meets the aiteria por this reaction Indicate which is neccessory the slow step the intermediates •J need these answer within 30 min. Thank Xou, Pleaze Consider g reguest myarrow_forwardThe gas-phase decomposition of di-tert-butyl peroxide. (CH₂) COOC(CH3)3, is first order in the temperature range 110°C to 280°C with a first- order rate coefficient k=3.2 x 10¹ exp[-(164 kJ mol/RT³ Part A What is the value of AH for this reaction in the temperature range between 110 Cand 280 C Express your answer with the appropriate units. ΔΗ" - 164 НА kJ mol Submit Previous Answers Request Answer Xncorrect; Try Again; 4 attempts remaining ?arrow_forwardThe elementary steps for a catalyzed reaction are shown below. Identify the catalyst? Identify the reactive intermediate? HyOy(0g) + I" (aq) —+ H_O(0) + IOT (sq) IO"(@a) + HyOz(0g) - HyO0+O2{0) +F(31) O The catalyst is H₂O2(aq); the reactive intermediate is 1(aq). Ob The catalyst is (aq); the reactive intermediate is 10"(aq). The catalyst is H₂O(); the reactive intermediate is 1" (aq). Od The catalyst is 1"(aq); the reactive intermediate is H₂O2(aq). The catalyst is 10(aq); the reactive intermediate is 1(aq).arrow_forward
- Given this reaction: ZNG) + 2HCI @q3 Hz (qD +Zn Cl2(aq) a) Descrive Hhe effect of increasing the cancentratian ag on the readtich rate cund response in terms of collisian theory |cdenti fy ane other Variable Hhat mignt affect fhe rate cand shoud be held constent dur ing this inestigation the wckependent and dependent vasi abre Of HCl jasti fy your c) ldentiey in this Thiestigationarrow_forwardIn a study of the gas phase decomposition of hydrogen peroxide at 400 °CH2O2(g)H2O(g) + ½ O2(g)the concentration of H2O2 was followed as a function of time.It was found that a graph of 1/[H2O2] versus time in seconds gave a straight line with a slope of 1.03 M-1 s-1 and a y-intercept of 13.4 M-1.Based on this plot, the reaction is (frist, second, zero)__________ order in H2O2 and the rate constant for the reaction is _____________ M-1 s-1.arrow_forwardThe rate constant for the reaction CH;Br + Cl- CH;CI + Br¯ in acetone is 5.9 × 10-3 L mol¬1 s-1. Is this reaction diffusion-controlled or limited by a large activa- tion energy?arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,

