
Concept explainers
(a)
Interpretation:
Line-angle structural formula has to be drawn for the given compound and the
Concept introduction:
In chemistry, structure is the arrangement of
In condensed structure representation,
- ✓ Some or all of the lines are omitted and atoms attached to carbon are written immediately after it.
In line-angle structure,
- ✓ Only shows bonds.
- ✓ C atoms assumed at each end and intersection of bonds and thus are not shown.
- ✓ H atoms are not shown.
- ✓ Assume 4 bonds to each C
- ✓ Fulfill C’s 4 bonds by adding hydrogens.
(b)
Interpretation:
Line-angle structural formula has to be drawn for the given compound and the functional group present on it has to be named.
Concept introduction:
In chemistry, structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
In condensed structure representation,
- ✓ Some or all of the lines are omitted and atoms attached to carbon are written immediately after it.
In line-angle structure,
- ✓ Only shows bonds.
- ✓ C atoms assumed at each end and intersection of bonds and thus are not shown.
- ✓ H atoms are not shown.
- ✓ Assume 4 bonds to each C
- ✓ Fulfill C’s 4 bonds by adding hydrogens.
Alcohol: It is an organic compound where it contains at least one
(c)
Interpretation:
Line-angle structural formula has to be drawn for the given compound and the functional group present on it has to be named.
Concept introduction:
In chemistry, structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
In condensed structure representation,
- ✓ Some or all of the lines are omitted and atoms attached to carbon are written immediately after it.
In line-angle structure,
- ✓ Only shows bonds.
- ✓ C atoms assumed at each end and intersection of bonds and thus are not shown.
- ✓ H atoms are not shown.
- ✓ Assume 4 bonds to each C
- ✓ Fulfill C’s 4 bonds by adding hydrogens.
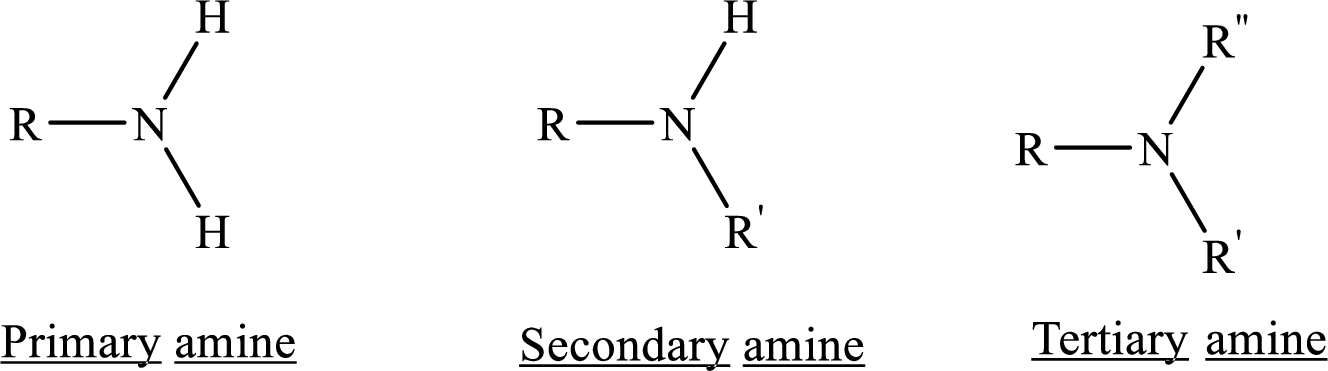
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.
(d)
Interpretation:
Line-angle structural formula has to be drawn for the given compound and the functional group present on it has to be named.
Concept introduction:
In chemistry, structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
In condensed structure representation,
- ✓ Some or all of the lines are omitted and atoms attached to carbon are written immediately after it.
In line-angle structure,
- ✓ Only shows bonds.
- ✓ C atoms assumed at each end and intersection of bonds and thus are not shown.
- ✓ H atoms are not shown.
- ✓ Assume 4 bonds to each C
- ✓ Fulfill C’s 4 bonds by adding hydrogens.
Ester: One
(e)
Interpretation:
Line-angle structural formula has to be drawn for the given compound and the functional group present on it has to be named.
Concept introduction:
In chemistry, structure is the arrangement of chemical bonds between atoms in a molecule, specifically which atoms are chemically bonded to what other atoms with what kind of chemical bond.
In condensed structure representation,
- ✓ Some or all of the lines are omitted and atoms attached to carbon are written immediately after it.
In line-angle structure,
- ✓ Only shows bonds.
- ✓ C atoms assumed at each end and intersection of bonds and thus are not shown.
- ✓ H atoms are not shown.
- ✓ Assume 4 bonds to each C
- ✓ Fulfill C’s 4 bonds by adding hydrogens.
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Chemistry In Context
- Check the box next to each molecule on the right that has the shape of the model molecule on the left: model 4 I 5 None of the above 6 7 I I 8 I molecules (check all that apply) H₂S H₂O HOCI BrCN X Start over You can drag the slider to rotate the model molecule.arrow_forwardWhat type(s) of intermolecular forces are expected between CH3CH,CH2CH2CH2CH3 molecules? H H H H H H- H- H. H H H H. Indicate with a Y (ves) or an N (no) which apply. dipole forces induced dipole forces hydrogen bonding Submit Answer Try Another Version 2 item attempts remainingarrow_forwardCheck the box next to each molecule on the right that has the shape of the model molecule on the left: model 2 3 4 I None of the above 6 17 8 I molecules (check all that apply) 0⁰3 NO3 H₂O* O NH3 X You can drag the slider to rotate the model molecule.arrow_forward
- Check the box next to each molecule on the right that has the shape of the model molecule on the left: 1 21 3 I model 4 51 None of the above 6 I 7 I molecules (check all that apply) NH3 2- SO 3 BrO3 + H₂O* X You can drag molecule.arrow_forwardNaming and Drawing Organic Molecules Recognizing different skeletal structures How many different molecules are drawn below? Explanation Check 000 G MacBook Air 1/5 Julianna V 2024 McGraw Hi LLC All Rights Reserved Terms of Use Privacy Center Accessibility F10 olo Ar ?arrow_forwardIII Naming and Drawing Organic Molecules Drawing a skeletal structure from a condensed structure Draw a skeletal ("line") structure of this molecule: CH3 CH3 C-CH2-O-CH2-OH CH₂ Click and drag to start drawing a structure. Explanation Check H σ Ö MacBook Air G È jn8iN2P19bv8M8v... 0 1/5 Julianna D 2024 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center | Accessibilityarrow_forward
- Write the systematic name of each organic molecule: structure CI CH3 CI C=CH-CH-CH3 CH3 CI CH=C CH3 CH3 CH3 CH3-CH-CH=C-CH3 Explanation Check L ୮ esc 80 F3 F5 name 5 ? MacBook Air 2024 McGraw Hill LLC. All Rights Reserved. Terms of Use | Privacy Center Accessibility F8 F10 000arrow_forwardCheck the box next to each molecule on the right that has the shape of the model molecule on the left: model 1 2 3 4 5 6 7 8 None of the above molecules (check all that apply) Х CH4 + NH CH3O ☐ CH3Cl You can drag the slider to rotate the model molecule. Note for advanced students: the length of bonds and size of atoms in the model is not necessarily realistic. The model is only meant to show you the general geometry and 3D shape of the molecule.arrow_forwardCheck the box next to each molecule on the right that has the shape of the model molecule on the left: model None of the above molecules (check all that apply) NO₂ imo, N₂H₂ CICN You can drag. the slider to rotate the model molecule.. 78哈日 ola A Rarrow_forward
- = O ORGANIC FUNCTIONAL GROUPS Naming alcohols Name these organic compounds: structure HO- CH₂ -CH₂-OH OH | CH3 -CH-CH₂-OH Explanation CH3 HOC-CH₂-OH CH3 Check X name 0 0 S MacBook Ⓒ2023 McGraw Hill LLC. All Rights Reserved. Terarrow_forwardDraw the condensed structure of 6,8-dimethyl-4-propyl-1-nonanol. Explanation Check Click anywhere to draw the first atom of your structure. Q Search © 2023 McGraw Hill LLarrow_forwardc) ОН d)arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





