
An adiabatic 0.2-m3 storage tank that is initially evacuated is connected to a supply line that carries nitrogen at 225 K and 10 MPa. A valve is opened, and nitrogen flows into the tank from the supply line. The valve is closed when the pressure in the tank reaches 10 MPa. Determine the final temperature in the tank (a) treating nitrogen as an ideal gas, and (b) using generalized charts. Compare your results to the actual value of 293 K.
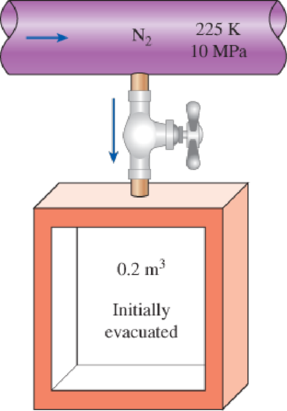
FIGURE P12–101
(a)
The final temperature in the tank by treating nitrogen as an ideal gas and compare the result to the actual value of
Answer to Problem 101RP
The final temperature in the tank by treating nitrogen as an ideal gas is
Explanation of Solution
Write the equation of mass balance.
Here, the inlet mass is
The change in mass of the system for the control volume is expressed as,
Here, the suffixes 1 and 2 indicates the initial and final states of the system.
Consider the given insulated tank as the control volume.
The valve is closed when the pressure in tank reaches to
Rewrite the Equation (I) as follows.
Write the energy balance equation.
Here, the heat transfer is
Since the tank is adiabatic, there is no heat transfer i.e.
The Equation (III) reduced as follows.
Substitute
Express the Equation (V) in molar basis.
Here, the molar mass of nitrogen is
Conclusion:
The inlet condition of the nitrogen is
While considering the nitrogen as the ideal gas, its enthalpy is solely depends on temperature.
Refer Table A-18, “Ideal-gas properties of nitrogen,
The molar enthalpy of nitrogen corresponding to the temperature of
Refer Equation (VI).
The final temperature of the nitrogen is expressed as follows.
Refer Table A-18, “Ideal-gas properties of nitrogen,
The final temperature
Thus, the final temperature in the tank by treating nitrogen as an ideal gas is
The percentage error with the actual temperature value of
The error associated is
(b)
The final temperature in the tank by using generalized departure charts.
Answer to Problem 101RP
The final temperature in the tank by using generalized departure charts is
Explanation of Solution
Refer Table A-1, “Molar mass, gas constant, and critical-point properties”.
The critical temperature and pressure of nitrogen gas is as follows.
The reduced pressure
At inlet:
Refer Figure A-29, “Generalized enthalpy departure chart”.
The enthalpy departure factor
Write formula for enthalpy departure factor
Here, the inlet molar enthalpy at ideal gas state is
Rearrange the Equation (I) to obtain
Write the formula for molar enthalpy at final state
Write the formula for molar internal energy at final state.
Here, the compressibility factor is
The universal gas constant
Conclusion:
Refer part (a) answer for
Substitute
Refer Equation (VI).
It is given that the actual final temperature of nitrogen is
Consider the exit temperature
The reduced pressure
Refer Figure A-29, “Generalized enthalpy departure chart”.
The enthalpy departure factor
Refer Figure A-15, “Nelson–Obert generalized compressibility chart”.
The compressibility factor
Refer Table A-18, “Ideal-gas properties of nitrogen,
The final molar enthalpy of nitrogen
Substitute
Substitute
Consider the exit temperature
The reduced pressure
Refer Figure A-29, “Generalized enthalpy departure chart”.
The enthalpy departure factor
Refer Figure A-15, “Nelson–Obert generalized compressibility chart”.
The compressibility factor
Refer Table A-18, “Ideal-gas properties of nitrogen,
The final molar enthalpy of nitrogen
Substitute
Substitute
Express interpolation formula to determine the final temperature
Substitute
Thus, the final temperature in the tank by using generalized departure charts is
The percentage error with the actual temperature value of
The error associated is
Want to see more full solutions like this?
Chapter 12 Solutions
Thermodynamics: An Engineering Approach
- Steam at 20 MPa and 620°C enters a steam turbine and expands to a condenser pressure of 100 kPa. An open feedwater heater is added operating at 2 MPa. Determine the fraction z of steam in decimals that leaves the turbine and goes to the open feedwater heater during the bleeding process. Please use the green book entitled "Thermodynamics Properties of Water Including Vapor, Liquid, and Solid Phases by Keenan, Keyes, Hill, and Moore for the steam table.arrow_forwardA frictionless piston fitted inside a cylinder containing gas at a pressure of 200 kPa, volume of 1 litre and temperature of 25 °C. Carbon monoxide is to be compressed in the system and the final volume is 0.5 litre. Work is done to move the piston and heat is transferred to the cooling system around the cylinder. Illustrate the importance of expressions for work done in a thermodynamic process by applying the first principles while calculating the work done when the expansion is ADIABATIC, ISOTHERMAL and POLYTROPIC for n=1.5.arrow_forwarda reciprocating compressor 0.1 m3 of air at 0.95 bar and 32°C is compressed according to PVn =constant until the pressure is 7 bar. Determine the volume and temperature of the air after compression. The work done on the air and the heat rejected to the cylinder walls assuming the compressor is water cooled and n=1.1 show the cancellation of units and formula usedarrow_forward
- 5. The two tanks (Tank A and Tank B) are connected by a valved tube. Initially, the valve is closed. Tank A contains 1 MPa, 1.5 kg of water vapor at 350°C, and tank B contains 3 kg of saturated water at 150°C and dryness x=0.5. The valve was opened and the water vapor moved and finally the pressure in the two tanks was equal to 300 kPa. Answer the following questions. (1) Calculate the volumes of tank A and tank B, respectively. (2) Calculate the final temperature and dryness (in case of saturation). (3) Find the amount of heat transfer during this process.arrow_forward(b) A rigid tank of 10 L vessel initially contains a mixture of liquid water and vapor at 100°C with 12.3% quality. Heat of 100 kJ is supplied to the rigid tank until the water reach superheated level at 1 MPa. The maximum temperature that the rigid tank can withstand is of 300°C. Evaluate if the rigid tank is able to withstand the heat supply of 100 kJ. Justify your answer with related calculation.arrow_forwardA and B containers (fixed volume) are connected together by a valve. Tank A contains 400 kPa and 0.3 m3 refrigerant 134a at 60% dryness. Tank B contains 0.5 m3 of refrigerant 134a at 240 kPa pressure and 100 oC temperature. Then the valve is opened and the system reaches 280 kPa equilibrium pressure. Calculate the final temperature.arrow_forward
- Find the dryness fraction of a steam having a specific volume of 15 m3 /kg after expansion in a turbine. The specific volumes of saturated liquid and saturated vapor corresponding to the pressure are 0.001 m3 /kg and 15.25 m3 /kg, respectively.arrow_forwardA well insulated piston-cylinder assembly contains one mole of ideal gas. There are two 3000 kg blocks on this well insulated 0.04 m2 piston. Its starting temperature is 600 K and ambient pressure is 4 bar. The gas is compressed by adding another 3000 kg block. Fixed volume heat capacity is given as (5/2) R.(a) Calculate the values of ∆Ssis and ∆Sis.(b) Does this process violate the second law of thermodynamics? Explain.arrow_forwardIt is insulated against heat, except for a cylinder base B with a volume of 100 liters. This cylinder is divided into two chambers by a heat-tight and frictionless piston. Compartment A contains 100 kPa pressure and 20 oC air, and compartment B contains neon gas at 30 oC. Initially, the volumes of both compartments are equal. Compartment A is connected to a pipe through which air flows at 800 kPa and 20 oC. The valve is opened and closed when the pressure in the chamber reaches 800 kPa. In compartment B, neon gas is inverted and the temperature changes in a steady state. a) Calculate the final volume and compression work of the neon. b) Calculate the temperature and mass of the air in compartment A in the final state. c) Calculate the total entropy change of the entire system.arrow_forward
- The internal energy of a certain ideal gas is given by the expression U=850 + 0.529pv Btu/lb Where p is in psia. Determine the exponent k in PVK=C for the gas undergoing an isentropic processarrow_forwardAn insulated evacuated tank of 1.75-m> volume is attached to a line containing steam at 400 Wa and 513.15 K (240°C). Steam flows into the tank until the pressure in the tank reaches 400 kPa. Assuming no heat flow from the steam to the tank, prepare graphs showing the mass of steam in the tank and its temperature as a function of pressure in the tankarrow_forwardCombustion gases enter the nozzle of a jet engine at 3.8 bar and 820°C. The nozzle is well insulated and the gases expand down to an exit pressure of 1.1 bar. Determine the temperature and the velocity of the gases at exit from the nozzle assuming the velocity at inlet is negligible. For the combustion gases takecp = 1.15 KJ/Kg. K and the ratio of specific heats = 1.32 show cancellation of units and formula usedarrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





