
Chemistry for Today: General, Organic, and Biochemistry
9th Edition
ISBN: 9781305960060
Author: Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 13, Problem 13.5E
Several important alcohols are well known by common names. Give a common
name for each of the following:
a.
b. 
c.
d. 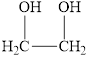
e. 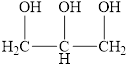
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
1. Using Br2 in C2H4Br2 will result in HBr and ______.
a. C2H3Cl3
b. C2H4Cl3
c. C2H2Cl3
d. none of the above
2. How many halogenation are posible in propane?
a. 3
b. 8
c. 6
d. 10
3.Sulfonation of pentane will result in ________ and water.
a. C5H11SO3H
b. C5H12SO3H
c. C5H14SO3H
d. none of the above
4.Nitration of hexane will result in ________ and water.
a. C6H13SO3H
b. C6H15NO2
c. C6H13NO2
d. C6H14NO2
5.How many moles of O2 in heating a C12H26 (dodecane)
a. 27
b. 37
c. 24
d. none of the above
1. An alkene reacts with water with an acid catalyst results into a formation of:
A. Aldehyde
B. Ketone
C. Alcohol
D. Ester
2. 3-Methylhexanal with K2Cr2O7 will yield:
A. 3-Methyl-1-hexanol
B. 3-Methylhexanoic acid
C. 3-Methyl-1-hexanone
D. 3-Methyl-1-hexanethiol
3. This is a reverse process of Hydration reaction:
A. Oxidation reaction
B. Reduction reaction
C. Dehydration reaction
D. Hydration reaction
4. Acetic acid reacts with a strong base forms:
A. Salt
B. Water
C. Salt and Water
D. No reaction
5. Ketones can be further oxidized with benedict's solution into:
A. Alcohol
B. Aldehyde
C. Catalysts
D. No reaction
H
H-C-OTC -C-
C-CH
n
HOMEWORK
1. Write a structural formula for each of the following:
a. An alcohol C3H80
b. An ether C4H100
c. An aldehyde C3H60
d. A ketone C3H60
e. A carboxylic acid C3H6O2
f. An ester CsH 10O2
2. Write an equation for the reaction of CH2=CHCH2CH3 with each of
the following reagents:
a. Hydrogen chloride
b. Hydrogen (Pt catalyst)
c. Ozone, followed by Zn, H*
d. BH3 followed by H2O2, OH
e. Bromine
f. H2О, H*
g. KMnO4, OH
h. Oxygen (Combustion)
Chapter 13 Solutions
Chemistry for Today: General, Organic, and Biochemistry
Ch. 13 - Draw general formulas for an alcohol and phenol,...Ch. 13 - Prob. 13.2ECh. 13 - Assign IUPAC names to the following alcohols: a....Ch. 13 - Assign IUPAC names to the following alcohols: a....Ch. 13 - Several important alcohols are well known by...Ch. 13 - Prob. 13.6ECh. 13 - Draw structural formulas for each of the...Ch. 13 - Draw structural formulas for each of the...Ch. 13 - Name each of the following as a derivative of...Ch. 13 - Name each of the following as a derivative of...
Ch. 13 - Prob. 13.11ECh. 13 - Draw structural formulas for each of the...Ch. 13 - Prob. 13.13ECh. 13 - Classify the following alcohols as primary,...Ch. 13 - Classify the following alcohols as primary,...Ch. 13 - Draw structural formulas for the four aliphatic...Ch. 13 - Why are the boiling points of alcohols much higher...Ch. 13 - Arrange the compounds of each group in order of...Ch. 13 - Prob. 13.19ECh. 13 - Draw structural formulas for the following...Ch. 13 - Prob. 13.21ECh. 13 - Draw the structures of the chief product formed...Ch. 13 - Draw the structures of the chief product formed...Ch. 13 - Draw the structures of the ethers that can be...Ch. 13 - Prob. 13.25ECh. 13 - Give the structure of an alcohol that could be...Ch. 13 - Give the structure of an alcohol that could be...Ch. 13 - What products would result from the following...Ch. 13 - What products would result from the following...Ch. 13 - Each of the following conversions requires more...Ch. 13 - Each of the following conversions requires more...Ch. 13 - The three-carbon diol used in antifreeze is It is...Ch. 13 - Methanol is fairly volatile and evaporates quickly...Ch. 13 - Prob. 13.34ECh. 13 - Prob. 13.35ECh. 13 - Name an alcohol used in each of the following...Ch. 13 - Prob. 13.37ECh. 13 - Prob. 13.38ECh. 13 - Assign a common name to each of the following...Ch. 13 - Assign a common name to each of the following...Ch. 13 - Assign the IUPAC name to each of the following...Ch. 13 - Assign the IUPAC name to each of the following...Ch. 13 - Prob. 13.43ECh. 13 - Draw structural formulas for the following: a....Ch. 13 - Prob. 13.45ECh. 13 - Prob. 13.46ECh. 13 - Prob. 13.47ECh. 13 - Arrange the following compounds in order of...Ch. 13 - Arrange the compounds in Exercise 13.48 in order...Ch. 13 - Prob. 13.50ECh. 13 - Complete the following reactions: a. b....Ch. 13 - Prob. 13.52ECh. 13 - Lipoic acid is required by many microorganisms for...Ch. 13 - Alcohols and thiols can both be oxidized in a...Ch. 13 - Prob. 13.55ECh. 13 - Prob. 13.56ECh. 13 - Prob. 13.57ECh. 13 - Thiols have lower boiling points and are less...Ch. 13 - Prob. 13.59ECh. 13 - Prob. 13.60ECh. 13 - Prob. 13.61ECh. 13 - Prob. 13.62ECh. 13 - A mixture of ethanol and 1propanol is heated to...Ch. 13 - Prob. 13.64ECh. 13 - Prob. 13.65ECh. 13 - Prob. 13.66ECh. 13 - Prob. 13.67ECh. 13 - Figure 13.8 points out that methanol is used as a...Ch. 13 - Figure 13.13 focuses on the use of thiol chemistry...Ch. 13 - Prob. 13.70ECh. 13 - Prob. 13.71ECh. 13 - Prob. 13.72ECh. 13 - The compound that has the greatest polarity is: a....Ch. 13 - Alcoholic beverages contain: a. wood alcohol. b....Ch. 13 - Prob. 13.75ECh. 13 - Which of the following compounds is an ether? a....
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Four test tubes containing different hydrocarbons: acetone, benzoic acid, cyclohexane, and ethylamine were tested for solubility with different solvents: H2O, dilute HCl solution, and a strong solution of NaOH. The results obtained from the solubility test were tabulated below. Determine the content of each test tube A, B, C, and D. Choices: A. Cyclohexane B. Ethylamine C. Benzoic Acid D. Acetonearrow_forward11. What is the major organic product obtained from the following reaction? -Br a. 1 b. 2 3 c. d NaOEt OH 2 COEtarrow_forward13. The ferric chloride (FeCb) solution is used as a test for: a. Alcohols d. Alkyl halides b. Phenols c. Carboxylic Acids e. Alkenesarrow_forward
- Isopropyl alcohol is Select one: a. CH3CH2OH b. CH3CH(OH)CH3 c. CH3CH2CH2OH d. CH3OHarrow_forward3. Write the condensed structural formula of the organic products for ethyl ethanoate when it reacts with each of the following: a. NaOH b. H2O and HCIarrow_forwardWhich of the following is least soluble in water? A. CH3OH B. CH3CH2CH2OH C. CH3CH2OH D. CH3CH2CH2CH2OH E. HOCH2OHarrow_forward
- Identify the following compound: CH3 H₂C HO- -CH3 a. ketone b. ester c. carboxylic acid Od. alcoholarrow_forwardWhich of the following is/are secondary (2°) alcohols? он он "HO. 1 A. only 3 B. only 2 and 4 C. 1, 2, 3 and 4 D. only 1arrow_forwards: On A.What is the condensed structural formula of: 1. Methanol 2. Benzoic Acid 3. Methyl Benzoate 4. 1- Pentanol 5. Acetic Acid 6. Pentyl acetate 7. 1-octanol 8. Octyl acetate 9. Benzylic alcohol 10. Benzyl acetate 11. 1-Propanol 12. Propyl acetate Accessibility: Good to go hparrow_forward
- 1. Rank the following with increasing acidity: CH3OH, HCl, NH3, and CH4 2. Select the correct molecule with the lowest surface tension. a. CH3CH2CH2OH b. CH3CH2CH2NH2 c. CH3CH2CH2CH3 d. CH3CH2CH2Clarrow_forward7. H3C-CH The compound above is classified as a(n) alkane d. ketone alkene a. e. b. carboxylic acid c. akdehyde 8. Which of the follow ing is a secondary alcohol? d. CH3OH a. H3C C CH3 CH3 b. H3C0-CH3 e. CH3CH2OH c. OH H3C CH3 H. 9. CH3 OH -CH-CH-CH, H3C What is the correct name for the above compound? a. 2-methyl-3-butanol b. 2-pentanol isobutanol d. 3-methy-2-butanol none of these e. c.arrow_forwardH. E. G но но HO B. C HO ONa HO 2.52 Rank molecules A-H in order from lowest to highest boiling point. 2.8 Ranking Boiling Points and Solubilities of Structurally Similar Compoundsarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY