
Interpretation:
The cubane,
Concept introduction:
The complex vibrations exhibit by the polyatomic molecule is known as normal modes of vibrations. The vibrational modes of a molecule are IR or Raman active. If a molecule has centre of symmetry, then the modes which are IR-active will be Raman inactive and the modes that are IR-inactive will be Raman active. The total number of vibrational degrees of freedom for nonlinear molecule is represented by
Answer to Problem 14.82E
The cubane,
Explanation of Solution
The structure of cubane is shown below.
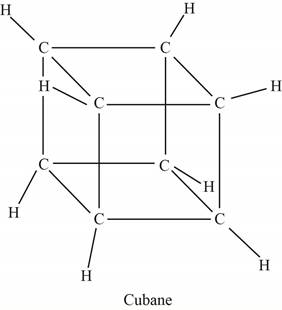
Figure 1
The point group of cubane is
The character table for point group
The expression
Similarly, the value of
The expression
The positive sign is taken for the proper rotation and negative sign is taken for improper axis.
Substitute the value of
Similarly, the value of
The expression
Substitute the value of
Similarly, the value of
The expression
The positive sign is taken for the proper rotation and negative sign is taken for improper axis.
Substitute the value of
Similarly, the value of
The expression
The positive sign is taken for the proper rotation and negative sign is taken for improper axis.
Substitute the value of
Similarly, the value of
The great orthogonality theorem for the reducible representation can be represented as,
Where,
•
•
•
•
•
The term
The irreducible representation for point group
Substitute the
Therefore, the cubane,
The cubane,
Want to see more full solutions like this?
Chapter 14 Solutions
Physical Chemistry
- Determine the number of total degrees of freedom and the number of vibrational degrees of freedom for the following species. a Hydrogen sulfide, H2S b Carbonyl sulfide, OCS c The sulfate ion, SO42 d Phosgene, COCl2 e Elemental chlorine, Cl2 f A linear molecule having 20 atoms g A nonlinear molecule having 20 atomsarrow_forwardWhich of the following molecules should have pure rotational spectra? a Dimethyltriacetylene, H3CCCCCCCCH3 b Cyanotetraacetylene, HCCCCCCCCCN Such molecules have been detected in intersteller space. c Nitric oxide, NO d Nitrogen dioxide, NO2 e Sulfur tetrafluoride, SF4 f Sulfur hexafluoride, SF6arrow_forwardWhich of the following molecules should have pure rotational spectra? a Deuterium, D2 D is 2H b Carbon monoxide, CO c cis1,2Dichloroethylene d trans1,2Dichloroethylene e Chloroform, CHCl3 f Buckminsterfullerene, C60arrow_forward
- Part D: Methane at room temperature At room temperature, the specific heat capacity (at constant volume) of methane is 27.7 How many vibrational modes are unfrozen in the average methane molecule at this temperature? Round your answer to the nearest whole number of modes. Hint: Remember that there are multiple types of modes contributing to the heat capacity at room temperature. mol *K' Part E: Carbon suboxide at low temperatures Carbon suboxide (C302) is a linear molecule. Determine its specific heat capacity (at constant volume) at very low temperatures. Enter the value in units of the universal gas constant R. Part F: Carbon suboxide at moderate temperatures Determine the specific heat capacity (at constant volume) of methane at moderate temperatures. Enter the value in units of the universal constant R.arrow_forwardWhat are the total number of degrees of freedom and the number of vibrational modes for ethanol C2H6O?arrow_forwardThe vibrational frequency of the ICl molecule is 1.15 ×1013 s-1. For every million (1.00 × 106) molecules in the ground vibrational state, how many will be in the first excited vibrational state at a temperature of 300 K?arrow_forward
- (c) Would you expect 109AgF to have a rotational constant that is higher,lower, or equal to that of 107AgF? Explain your reasoningarrow_forward1. Determine the number of translational, vibrational, and rotational degrees of freedom available to the molecule NH3. 2. Calculate the rotational constant (B) for the molecule H12C14N, given that the H-C and C-N bond distances are 106.6 pm and 115.3 pm respectively.arrow_forwardThis question pertains to rotational spectroscopy. Which of the following molecules would have a pure rotational spectrum and why? HCl, N2O, O3, SF4 What information is obtained from the rotational spectrum of a diatomic molecule and how can it be used to determine the bond length of a diatomic molecule? What is the selection rule for rotational spectroscopy? The rotational constant of 127I35Cl is 3.424 GHz. Calculate the ICl bond length.arrow_forward
 Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning

