
(a)
Interpretation:
The synthesis of given Grignard reagent from an alkyl, alkenyl, alkynyl, or aryl halide is to be suggested.
Concept introduction:
Grignard reagent can be prepared from an alkyl, alkenyl, alkynyl, or aryl halide where the
Answer to Problem 19.38P
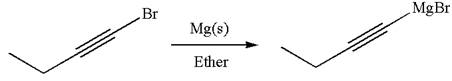
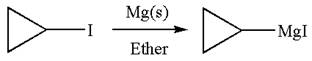
The synthesis of the given Grignard reagent from an alkynyl halide is:
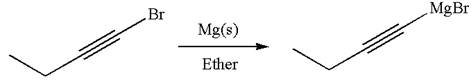
Explanation of Solution
The given Grignard reagent is:
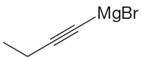
The given Grignard reagent can be prepared from alkynyl bromide as
The synthesis of the given Grignard reagent is provided by identifying alkynyl halide.
(b)
Interpretation:
The synthesis of given Grignard reagent from an alkyl, alkenyl, alkynyl, or aryl halide is to be suggested.
Concept introduction:
Grignard reagent can be prepared from an alkyl, alkenyl, alkynyl, or aryl halide where the
Answer to Problem 19.38P
The synthesis of given Grignard reagent from an
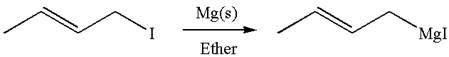
Explanation of Solution
The given Grignard reagent is:
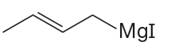
The given Grignard reagent can be prepared from alkyl iodide as
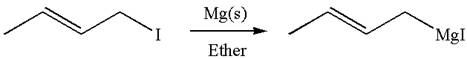
The synthesis of given Grignard reagent is provided by identifying alkyl halide.
(c)
Interpretation:
The synthesis of given Grignard reagent from an alkyl, alkenyl, alkynyl, or aryl halide is to be suggested.
Concept introduction:
Grignard reagent can be prepared from an alkyl, alkenyl, alkynyl, or aryl halide where the
Answer to Problem 19.38P
The synthesis of given Grignard reagent from an aryl halide is:
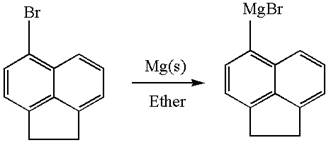
Explanation of Solution
The given Grignard reagent is:
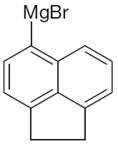
The given Grignard reagent can be prepared from aryl bromide as

The synthesis of the given Grignard reagent is provided by identifying aryl halide.
(d)
Interpretation:
The synthesis of given Grignard reagent from an alkyl, alkenyl, alkynyl, or aryl halide is to be suggested.
Concept introduction:
Grignard reagent can be prepared from an alkyl, alkenyl, alkynyl, or aryl halide where the
Answer to Problem 19.38P
The synthesis of the given Grignard reagent from an aryl halide is:
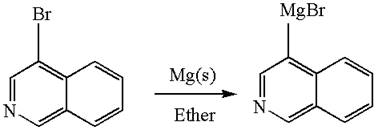
Explanation of Solution
The given Grignard reagent is:
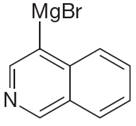
The given Grignard reagent can be prepared from aryl bromide as
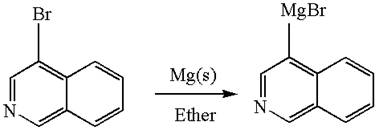
The synthesis of given Grignard reagent is provided by identifying the aryl halide.
(e)
Interpretation:
The synthesis of given Grignard reagent from an alkyl, alkenyl, alkynyl, or aryl halide is to be suggested.
Concept introduction:
Grignard reagent can be prepared from an alkyl, alkenyl, alkynyl, or aryl halide where the
Answer to Problem 19.38P
The synthesis of given Grignard reagent from an alkyl halide is:
Explanation of Solution
The given Grignard reagent is:
![]()
The given Grignard reagent can be prepared from alkyl iodide as
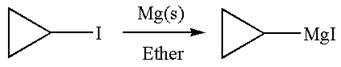
The synthesis of given Grignard reagent is provided by identifying alkyl halide.
(f)
Interpretation:
The synthesis of given Grignard reagent from an alkyl, alkenyl, alkynyl, or aryl halide is to be suggested.
Concept introduction:
Grignard reagent can be prepared from an alkyl, alkenyl, alkynyl, or aryl halide where the
Answer to Problem 19.38P
The synthesis of the given Grignard reagent from an aryl halide is:
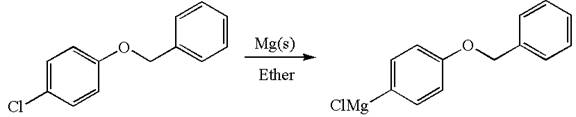
Explanation of Solution
The given Grignard reagent is:
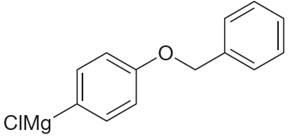
The given Grignard reagent can be prepared from aryl chloride as

The synthesis of given Grignard reagent is provided by identifying aryl halide.
Want to see more full solutions like this?
Chapter 19 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





