
Biochemistry: Concepts and Connections (2nd Edition)
2nd Edition
ISBN: 9780134641621
Author: Dean R. Appling, Spencer J. Anthony-Cahill, Christopher K. Mathews
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2, Problem 20P
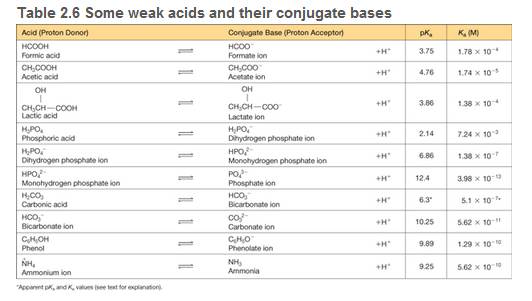
A biochemical reaction takes place in a 1.00 ml solution of 0.0250 M phosphate buffer initially at pH = 7.20 (see Table 2.6 for pkas of phosphate species).
a. Are the concentrations of any of the four possible phosphate species negligible? If so, identify them and explain your answer.
b. During the reaction, 3.80 µmol of HCI are produced. Calculate the final pH of the reaction solution. Assume that the HCI is completely neutralized by the buffer.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
A monoprotic weak acid, HA, dissociates in water according to the reaction
HA(aq) = H*(aq) + A¯(aq)
The equilibrium concentrations of the reactants and products are [HA] = 0.290 M, H+| = 3.00 × 10¬4 M, and
[A-] = 3.00 x 10-4 M. Calculate the value of pKa for the acid HA.
pKa
Identify the acid and conjugate base in each reaction. Calculate the pKa
for each acid. List them in order from the strongest to weakest acid. The
acid-ionization constants, Ka, at 25°C are listed for each.
a. HC2H3O2 + H2O ↔ H3O+ + C2H3O2-acetic acid, KA = 1.7 x 10-5
b. HC7H5O2 + H2O H₂O+ + C7H5O2-benzoic acid, KA= 6.3 x 10-5
c. HC6H4NO2 + H2O ↔ H3O++ C6H4NO2-nicotinic acid, KA =
1.4 x 10-5
A monoprotic weak acid, HA, dissociates in water according to the reaction
HA(aq) = H+ (aq) + A¯(aq)
The equilibrium concentrations of the reactants and products are [HA] = 0.220 M, [H+] = 3.00 × 10−4 M, and
[A¯] = 3.00 × 10−4 M. Calculate the value of pKa for the acid HA.
pKa
=
Chapter 2 Solutions
Biochemistry: Concepts and Connections (2nd Edition)
Ch. 2 - Suppose a chloride ion and a sodium ion are...Ch. 2 - Draw two different possible hydrogen-bonding...Ch. 2 - Prob. 3PCh. 2 - 4. What is the pH of each of the following...Ch. 2 - Prob. 5PCh. 2 - The weak acid HA is 2% ionized (dissociated) in a...Ch. 2 - 7. Calculate the pH values and draw the titration...Ch. 2 - What is the pH of the following buffer mixtures?...Ch. 2 - a. Suppose you wanted to make a buffer of exactly...Ch. 2 - Prob. 10P
Ch. 2 - You need to make a buffer whose pH is 7.0, and you...Ch. 2 - Describe the preparation of 2.00 L of 100 glycine...Ch. 2 - Carbon dioxide is dissolved in blood (pH 7.4) to...Ch. 2 - What is the molecular basis for the observation...Ch. 2 - The anno acid arginine ionizes according to the...Ch. 2 - It is possible to make a buffer that functions...Ch. 2 - A student is carrying out a biological preparation...Ch. 2 - Histidine is an amino acid with three titratable...Ch. 2 - Prob. 19PCh. 2 - A biochemical reaction takes place in a 1.00 ml...Ch. 2 - Is RNA-binding enzyme RNase A more likely to have...Ch. 2 - Consider a protein in which a negatively charged...Ch. 2 - Prob. 23PCh. 2 - Prob. 24P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biochemistry and related others by exploring similar questions and additional content below.Similar questions
- Describe reaction between AP and PNPP and how is used to determine the kinetic parameters of AP. i. İndicate the value for the activity of AP(which is 3.08) What does it mean? Is your enzyme active or not? What does it say about the purity of your enzyme? ii. How the purification process affects the activity?arrow_forwardGiven the titration curve of the hypothetical polyprotic acid X at 0.100 M concentration (pKa1=4.0, pKa2=8.0, pKa3=12.0) titrated with 0.600 M NaOH, identify the pH at point C, H, E, and M.arrow_forwardThe reaction quotient is Q=1.6×10-26 Part B What pH is needed to produce this value of Q if the concentration and pressure values are [Br2]=2.50×10−4M , [Br−]=11.65M, [SO42−]=9.50M, and PSO2=3.50×10−5atm ? Express your answer numerically to two decimal places.arrow_forward
- Consider a buffer solution of acetate. The volume is 500 ml, the concentration is 200 mM, and the pH is 5.0. a. How many total moles of acetate plus acetic acid are present in the solution? Express answer as x.y with one digit before and one after the decimal place. b. What is the ratio of acetate ions (Ac-) to acetic acid ions (HAc) in the buffer solution (pH 5.0) if the pka is 4.76? Express the answer as x.yz with one digit before and two after the decimal place. c. How many moles of acetate are present in the solution? Express your answer to one decimal place. d. How many moles of acetic acid are present in the solution? Express your answer to one decimal place.arrow_forwardThe co-amoxiclav disc (20/10) has two drugs present, amoxicillin and clavulanate. The purpose of clavulanate is best described by which ONE of the following? Select one: A. To increase the solubility of amoxicillin B. To stabilise amoxicillin from spontaneous hydrolysis in aqueous solution C. To act synergistically with amoxicillin by inhibiting penicillinases produced by the organism D. To promote increased amoxicillin transport across the outer membrane of the organism E. To promote increased binding affinity of amoxicillin with the bacterial targetarrow_forwardA simple biochemical reaction with three molecules has solutions that oscillate toward a steady state when positive constants a and b are below the curve b - a = (b + a)3. Find the largest possible value of a for which the reaction has solutions that oscillate toward a steady state. (Hint: Find where da/db = 0. Derive values for a + b and a - b, and then solve the equations in two unknowns.) Source: Mathematical Biology.arrow_forward
- Highlight your values of A,B,C and D. For your question: A mL of B mol/L sodium phosphate solution is combined with C mL of D mol/L calcium bicarbonate. (Calcium bicarbonate is soluble.) A mL B mol/L C mL D mol/L 75.0 ml 0.300 67.5 0.350 Before you begin your reaction, you must accurately produce 1.500 L of your sodium phosphate solution from sodium phosphate trihydrate solid. Write out a procedure to explain all the steps you will take in the lab when making the solution to ensure that your solution concentration is accurate. Please include calculations that show the required mass of solid. Also include the correct names of all equipment used.arrow_forwardWhat volume of 0.1M NaOH (reagent) needs to be added to increase your 7.5 pH (buffer) by 0.5 pH units. The goal is to further purify MOPs (see image). Protonated form = 6.6 x 10.3^-3 mol/L Deprotonated form = 1.32 x 10^-2 mol/L Show calculations.arrow_forwardIn your acid phosphatase enzyme kinetics lab, you constructed a Lineweaver-Burk plot. Lefs assume that you graphicaly obtain 1/Vmax - 9.33 (umoliey and -1Km--0.012 M. Which of the following is correct? A) Vmax = 9.33 (no units) B) Km = 0.012 (no units) C) Vmax = (1/Vru" = 0.107 umolis D) Km =-14-1K = 83.3 pM E) Both C) and D) are correctarrow_forward
- Table 2: Effect of pH on Enzyme Activity pH Absorbance 2 0.05 4 0.35 6 0.8 8 0.5 10 0.4 12 0.1 Use the above data table to complete the following questions: a) Plot the data “pH Vs Abs” using “connect the data point type of graph”. Label the graph with dependent and independent variables where they should be. Provide a title for the graph. b) Over what pH range does catechol oxidase catalyze catechol to benoquinone? c) Explain why the graph has a bell-shaped curve.arrow_forwardPotentiometric titration curve is given below, which is obtained during the potentiometric titration between strong base KOH (0.2 M) with strong acid HI, label the point in the curve from the following options. If more than one points are present than write as x, y(means separate by using comma) a)The point where pH is because of excess OH - ions. b) The point where pH is only because of HI in water. c)The point where [HI]= [I] in water. d)The point where pH=pka e) The point where all HI is neutralized. f) The point where pH corresponds to solution of [I- ] in water. 14 13 12 11 10 9 pH 6. TITIT TITarrow_forwardA researcher is preparing a reaction mixture to test the activity of a protein. They combine the required reaction components, which contained in a final 100 ml reaction volume 200 mM NaCl, unknown concentrations of acetic acid and acetate anions and a total [H+] concentration of 790 ricromolar. Can you determine the pH of the solution? Provide the answer to one decimal place. Note: You may need to round the numbers to get the required answer. 100 Strips pH indicator strips non-bleeding pH pH 0-14 EM-Reagents Dip in-read while still moist. immerse in weakly-buffered solutions une there is no further color change (1-10 min) 3 7 5 6arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman
BiochemistryBiochemistryISBN:9781319114671Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.Publisher:W. H. Freeman Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman
Lehninger Principles of BiochemistryBiochemistryISBN:9781464126116Author:David L. Nelson, Michael M. CoxPublisher:W. H. Freeman Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY
Fundamentals of Biochemistry: Life at the Molecul...BiochemistryISBN:9781118918401Author:Donald Voet, Judith G. Voet, Charlotte W. PrattPublisher:WILEY BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305577206Author:Reginald H. Garrett, Charles M. GrishamPublisher:Cengage Learning Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON
Fundamentals of General, Organic, and Biological ...BiochemistryISBN:9780134015187Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. PetersonPublisher:PEARSON

Biochemistry
Biochemistry
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:9781464126116
Author:David L. Nelson, Michael M. Cox
Publisher:W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul...
Biochemistry
ISBN:9781118918401
Author:Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:WILEY

Biochemistry
Biochemistry
ISBN:9781305961135
Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:Cengage Learning

Biochemistry
Biochemistry
ISBN:9781305577206
Author:Reginald H. Garrett, Charles M. Grisham
Publisher:Cengage Learning

Fundamentals of General, Organic, and Biological ...
Biochemistry
ISBN:9780134015187
Author:John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:PEARSON
Enzyme Kinetics; Author: MIT OpenCourseWare;https://www.youtube.com/watch?v=FXWZr3mscUo;License: Standard Youtube License