
Concept explainers
Interpretation:
The validation corresponding to the fact that data of carbon monoxide coverage and pressure follows a Langmuir isotherm is to be stated. The equilibrium constant for the adsorption process is to be stated.
Concept introduction:
The isotherm model that describes the adsorption process, if an adsorbate acts as an ideal gas at the isothermal situation is known as Langmuir adsorption isotherm model. In the given isothermal situation, the partial pressure of the adsorbate is related to the volume of the adsorbate which is adsorbed on the solid adsorbent.
Answer to Problem 22.47E
The occurrence of the linear straight line between
The equilibrium constant for the adsorption process is
Explanation of Solution
The given temperature is
The given data of carbon monoxide coverage and pressure in torr is mentioned in the table below.
| Coverage | Pressure (torr) |
The concentration of carbon monoxide is calculated by the formula given below.
Where,
•
•
•
•
•
•
The value of universal gas constant is
| Pressure | Coverage | |||
The graph between
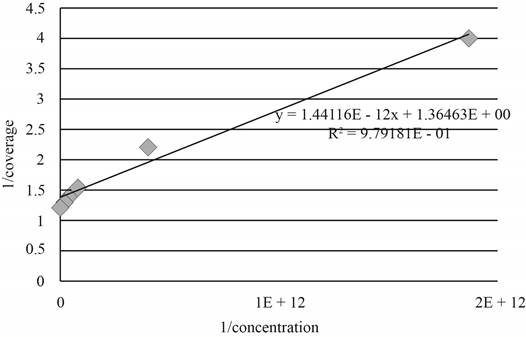
Figure 1
As, the plot between
The value of slope is predicted as
The value of equilibrium constant is calculated by the expression given below.
Substitute the value of slope in the above expression.
Therefore, the value of equilibrium constant is
The given data of carbon monoxide coverage and pressure follows a Langmuir isotherm.
The equilibrium constant for the adsorption process is
Want to see more full solutions like this?
Chapter 22 Solutions
Physical Chemistry
- Sodium hydride (NaH) crystallizes in the rock-salt structure, with four formula units of NaH per cubic unit cell. A beam of monoenergetic neutrons, selected to have a velocity of 2.639 × 103 ms-1 , is scattered in second order through an angle of 2u= 36.26° by the parallel faces ofthe unit cells of a sodium hydride crystal.(a) Calculate the wavelength of the neutrons.(b) Calculate the edge length of the cubic unit cell.(c) Calculate the distance from the center of an Na+ ion to the center of a neighboring H- ion.(d) If the radius of an Na+ ion is 0.98 Å, what is the radius of an H- ion, assuming the two ions are in contact?arrow_forwardEstimate the density of calcium oxide (rock salt structure), knowing that the ion rays are 100 pm and 140 pm for calcium and oxygen, respectively.arrow_forward12 Calculate the density of a metal (in g cm3 with two decimal places) that has the energy of vacancy formation of 50.1 kJ mol 1 and equilibrium number of vacancies of 1.70 × 1026 vacancies m3 at 700 °C. The atomic weight is given as 52.00 g mol-¹. Gas constant = 8.314 J mol-1 K-1. Avogadro's number = 6.022 x 1023 atoms mol-1. Type your answer...arrow_forward
- One of the chemical controversies of the nineteenth century concerned the element beryllium (Be). Berzelius originally claimed that beryllium was a trivalent element (forming Be3+ ions) and that it gave an oxide with the formula Be2O3. This resulted in a calculated atomic mass of 13.5 for beryllium. In formulating his periodic table, Mendeleev proposed that beryllium was divalent (forming Be2+ ions) and that it gave an oxide with the formula Be2O3. This assumption gives an atomic mass of 9.0. In 1894, A. Combes (Comptes Rendus 1894, p. 1221) reacted beryllium with the anion C5H7O2and measured the density of the gaseous product. Combess data for two different experiments are as follows: I II Mass 0.2022 g 0.2224 g Volume 22.6 cm3 26.0 cm3 Temperature 13C 17C Pressure 765.2 mm Hg 764.6 mm If beryllium is a divalent metal, the molecular formula of the product will be Be(C5H7O2)2; if it is trivalent, the formula will be Be(C5H7O2)3. Show how Combess data help to confirm that beryllium is a divalent metal.arrow_forwardWhat is the equilibrium number of vacancies, per cubic metre, for copper at 628 °C. Given: Activation energy per vacancy = 0.8 eV/atom Atomic weight of copper = 58.5 g/mol and density at the given temperature is 8.40 g/cm³ Boltzmann's constant kg= 1.38×10-23 J/atom.K = 8.62x10-5 eV/atom.K Your answer should be in scientific notation with 5 significant figures. The following example shows the format in which you should input your answer: 1.2475e8. Useful formulae: NAA density (p) = V.Na where Avagadro's number Na= 6.022 x 1025 atoms/mol Np Qv = exp kBTarrow_forwardWhy do high pressures favor the formation of the diamond from graphite?arrow_forward
- Calculate the number of Frenkel defects per cubic meter in potassium chloride at 500°C. The energy required to form each Frenkel defect is 2.6 eV, whereas the density for KCl is 1.955 g/cm3.arrow_forwardThe Haber process is very important for agriculture because it converts N2(g) from the atmosphere into bound nitrogen, which can be taken up and used by plants. The Haber process reaction is N2(g) + 3 H2(g) = 2 NH3(g). The reaction is exo- thermic but is carried out at relatively high temperatures. Why?arrow_forwardGases produced by a chemical reaction can easily be collected over water. To determine the pressure of the dry gas, the vapor pressure of the water at that temperature must be subtracted from the total pressure. 1) Consider the following reaction: Mg(s) + 2 HC1(aq) → MgCl2(aq) + H2(g) The total pressure of gas collected over water is 725.0 mmHg and the temperature is 19.5°C What is the pressure of hydrogen gas formed in mmHg? Pressure = mmHgarrow_forward
- At its critical point, ammonia has a density of 0.235 g cm-3 .You have a special thick-walled glass tube that has a10.0-mm outside diameter, a wall thickness of 4.20 mm, anda length of 155 mm. How much ammonia must you sealinto the tube if you wish to observe the disappearance of themeniscus as you heat the tube and its contents to a temperature higher than 132.23°C, the critical temperature?arrow_forwardPolonium is the only element known to crystallize in the simple cubic lattice. (a) What is the distance between nearest neighbor polo- nium atoms if the first-order diffraction of X-rays with A = 1.785 Å from the parallel faces of its unit cells appears at an angle of 20 = 30.96° from these planes? (b) What is the density of polonium in this crystal (in g cm-3)?arrow_forwardCalculate the density of cesium if it crystallizes in the bcc structure with an atomic radius of 272 pm.arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning




