
a)
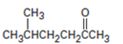
Interpretation:
The
Concept introduction:Acetoacetic ester synthesis converts an alkyl halide in to a methyl
To state:The alkyl halide which can be used to prepare 5-methyl-2-hexanone by acetoacetic ester synthesis.
b)
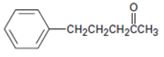
Interpretation:
The alkyl halide which can be used to prepare 5-phenyl-2-pentanone by acetoacetic ester synthesis is to be stated.
concept introduction: Acetoacetic ester synthesis converts an alkyl halide in to a methyl ketone having three more carbons. The methyl ketone part comes from acetoacetic eater while the remaining carbon comes from the alkyl halide. The reaction occurs in three steps 1) enolate ion formation ii) attack of the enolate anion on the alkyl halide iii) hydrolysis and decarboxylation.
To state: The alkyl halide which can be used to prepare 5-phenyl-2-pentanone by acetoacetic ester synthesis.
Trending nowThis is a popular solution!

Chapter 22 Solutions
Organic Chemistry
- apredict the product of the following reactions of aldehyde and ketonearrow_forwardIndicate how the following compounds can be synthesized from cyclohexanone and any other necessary reagents:arrow_forwardWhat products are formed in the base hydrolysis of the ester shown below with NaOH? Sodium ethanoate and ethanol B) Ethanol and ethanoic acid Ethanoic acid and sodium ethoxide Sodium ethanoate and water Methanol and sodium propanoate CH, C-O-CH2-CH,arrow_forward
- Name the carbonyl compound that would be formed by the complete acidic hydrolysis of the following hemiacetal/hemiketal or acetal/ketal: OH OCH₂CH₂CH₂CH₂CH3arrow_forwardHow could each of the following compounds be prepared from cyclohexanone?arrow_forwardHow would you synthesize diketones starting from acetoacetic ester?arrow_forward
- Name the following compound: O Butyl cyclohexanoate O Butanoic cyclohexanecarboxylic anhydride O Cyclohexyl butanoate O Cyclohexanoic butanoate O Butyl cyclohexyl anhydridearrow_forwardHow would you prepare the following estersarrow_forwardWhich of these is the most viable route for the preparation or synthesis of a carboxylic acid? O Alpha-carbon substitution of ketones O Oxidation of aldehydes O Nucleophilic acyl substitution of amides O Elimination of alcohols O Nucleophilic addition of ketonesarrow_forward
- What product is formed from the reaction of p-methylphenol with benzenediazonium chloride?arrow_forwardOne frequently used method for preparing methyl esters is by reaction of carboxylic acids with diazomethane, CH2N2. The reaction occurs in two steps: (l) protonation of diazomethane by the carboxylic acid to yield methyldiazonium ion, CH3N2+, plus a carboxylate ion; and (2) reaction of the carboxylate ion with CH3N2+. (a) Draw two resonance structures of diazomethane, and account for step 1. (b) What kind of reaction occurs in step 2?arrow_forwardWhat reagents are needed to carry out the following syntheses?arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


