
Concept explainers
- a. The isoelectric point (pl) of phenylalanine is pH 5.5. Draw the structure of the major form of phenylalanine at pH values of 1, 5.5, and 11.
- b. The isoelectric point of histidine is pH 7 6. Draw the structures of the major forms of histidine at pH values of 1, 4, 7.6, and 11. Explain why the nitrogen in the histidine ring is a weaker base than the α-amino group.
- c. The isoelectric point of glutamic acid is pH 3.2. Draw the structures of the major forms of glutamic acid at pH values of 1,3.2, 7, and 11. Explain why the side-chain
carboxylic acid is a weaker acid than the acid group next to the α-carbon atom.
(a)
Interpretation:
The structures of the major form of phenylalanine at
Concept introduction:
The more acidic strength increases the formation of the hydrogen ions and decreases the
Answer to Problem 24.27SP
The structures of the major form of phenylalanine at
Explanation of Solution
The structures of the major forms of phenylalanine at
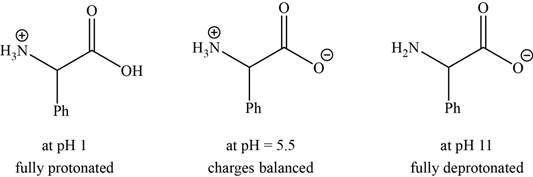
Figure 1
It is conferred from the above reaction that phenylalanine at
(b)
Interpretation:
The structures of the major forms of histidine at
Concept introduction:
The more acidic strength increases the formation of the hydrogen ions and decreases the
Answer to Problem 24.27SP
The structures of the major forms of histidine at
Explanation of Solution
The structures of the major forms of histidine at
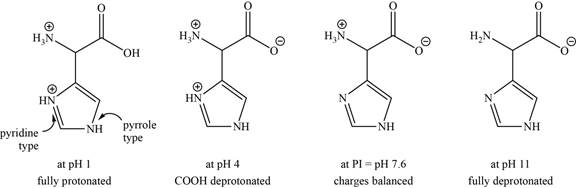
Figure 2
It is conferred from the above reaction that histidine at
(c)
Interpretation:
The structures of the major forms of glutamic acid at
Concept introduction:
The more acidic strength increases the formation of the hydrogen ions and decreases the
Answer to Problem 24.27SP
The structures of the major forms of glutamic acid at
Explanation of Solution
The structures of the major forms of glutamic acid at
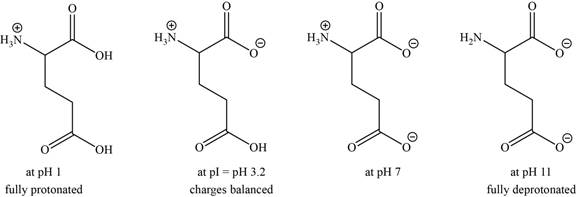
Figure 3
It is conferred from the above reaction that glutamic acid at
Want to see more full solutions like this?
Chapter 24 Solutions
Organic Chemistry (9th Edition)
- A recent article reported on the development of a new type of opioid drug that binds to a specific protein, but may not have the deleterious side-effects of many opioid drugs currently in use. The new drug is: N- N. The drug binds relatively tightly to the protein at low pH (Kd = 2 nM at pH = 5.5). The drug binds less tightly to the protein at higher pH (Kd = 18 nM at pH 7.4). Consider the drug-binding site in the protein. Explain briefly what aspect of the structure of the binding site could account for this result.arrow_forwardGT 2 A. Draw the structure of L-valine in a strongly basic B. What is the charge of this amino solution. acid in a strongly basic solution? В. A. What is the pH of D at its isoelectric point? Show the structure of this amino acid at its isoelectric point. C. What is the charge of this amino acid in a strongly acidic solution?arrow_forwardDraw the basic form of an amino acid. Why is it called an amino acid?arrow_forward
- Explain the differences in the litmus paper test results of the aqueous amino acid samples tested. a. alanine b. glutamic acid c. argininearrow_forwardDraw the structure of alanine in a solution at pH = 0arrow_forwardTetrapeptide example: C-A-F-E. 1. What is the structure of the tetrapeptide at pH 1.3. What is its charge at this point? 2. What is the structure of the tetrapeptide at pH 8.2. What is its charge at this point? 3. What is the structure of the tetrapeptide with 0 net charge. What is its isoelectric point?arrow_forward
- 4. The amino acid Asp189 lies at the base of the substrate specificity pocket in the enzyme trypsin. a. How is this related to typsin’s substrate specificity? Briefly describe the interactions between the substrate and the Asp 189. b. If the Asp189 was replaced with a lysine residue, how would this affect substrate specificity? c. The scientists that actually mutated the Asp189 to a lysine analyzed the three-dimensional structure of the enzyme and found that the lysine is actually not located in the specificity pocket. Instead, the Lys side chain reaches out of the base of the pocket, rendering the pocket nonpolar. With this additional information, determine how the substrate specificity would differ in the lysine-mutated enzyme.arrow_forward5. A new amino acid is discovered with the following structure: NH,CH,CHCOOH Into what category does this amino acid fit? Briefly explain. NH, 6. Draw the structure of the dipeptide arg-glu at a very acidic pH. 7. Write the products for the complete hydrolysis of CH₂ CH, CH₂ NH,CHCNHCHCNHCHCOOH 11 0 0 8. What is the amino acid sequence of a heptapeptide that, upon hydrolysis, yields the tripeptides Gly-Phe-Leu, Phe-Ala- Gly, and Leu-Ala-Tyr? The heptapeptide contains one residue each of Gly, Leu, and Tyr and two residues each of Ala and Phe. COOHarrow_forwardWhich of the following amino acids has a net negative charge at pH 7.0? A. glycine B. threonine C. aspartate D. argininearrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning





