
Concept explainers
(a)
Interpretation:
Given compound has to be prepared by Heck reaction using methyl-2-propenaote as the starting material.
Concept Introduction:
The
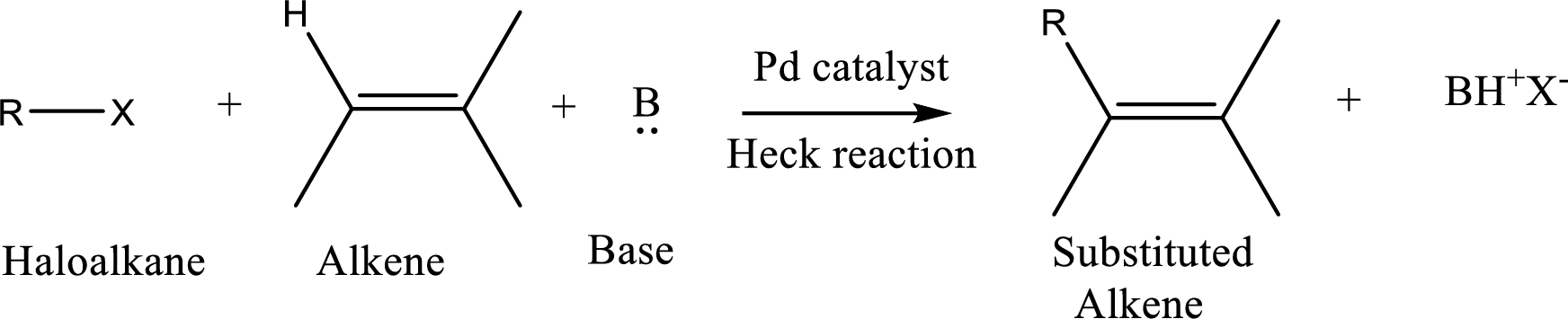
Reaction is highly regioselective in which formation of a new carbon-carbon bond occurs at a less substituted carbon of a double bond.
(b)
Interpretation:
Given compound has to be prepared by heck reaction using methyl-2-propenaote as the starting material.
Concept Introduction:
The
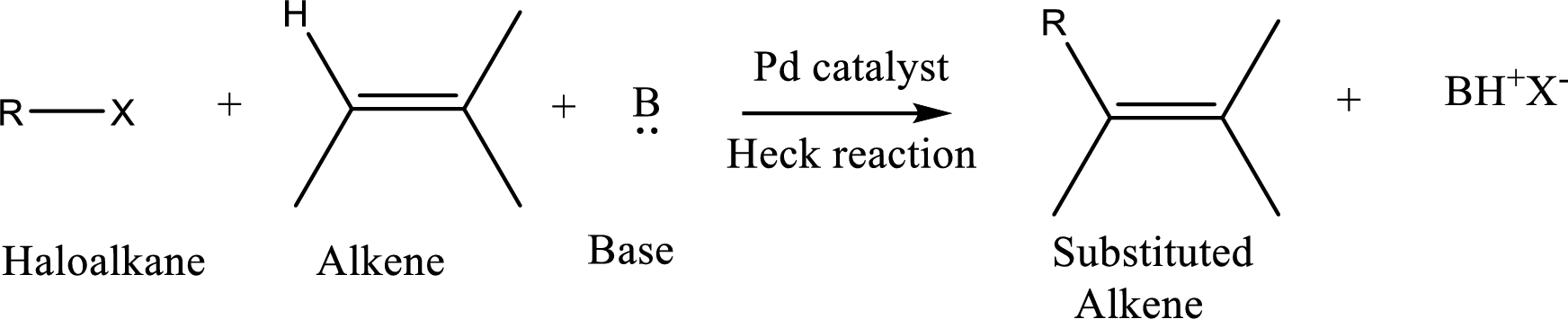
Reaction is highly regioselective in which formation of a new carbon-carbon bond occurs at a less substituted carbon of a double bond.
Trending nowThis is a popular solution!

Chapter 24 Solutions
Organic Chemistry
- (a) (CH3)3CBr give the common name (if possible).arrow_forward1. (a) Describe aromaticity, Kekule structure and resonance structure for benzene. (b) Why is benzene more stable than aliphatic alkenes?arrow_forward(i) State reagents G and J. (ii) Draw the structural formula for compounds D, E and H.arrow_forward
- Provide IUPAC names for the following compounds. (a) -OH (b)arrow_forward6. Describe concisely a chemical test to distinguish between the following pairs of compounds. (a) n-pentanol and 3-methylpentan-3-ol (b) Ethanal dan pentanal (c) Phenol and benzoic acidarrow_forwardDescribe how would you distinguish the following pairs, (a) Benzene and cyclohexane (b) Phenol and toluene (c) Phenol and benzoic acidarrow_forward
- (a) Although phenoxide ion has more number of resonating structures than carboxylate ion, carboxylic acid is a stronger acid than phenol. Give two reasons.(b) How will you bring about the following converstions?(i) Propanone to propane (ii) Benzoyl chloride to benzaldehyde(iii) Ethanal to but-2-enalarrow_forward(a) How will you convert:(i) Benzene to acetophenone (ii) Propanone to 2-Methylpropan-2-ol(b) Give reasons :(i) Electrophilic substitution in benzoic acid takes place at meta position.(ii) Carboxylic acids are higher boiling liquids than aldehydes, ketones and alcohols of comparable molecular masses.(iii) Propanal is more reactive than propanone in nucleophilic addition reactions.arrow_forwardPredict the major products formed when benzoyl chloride (PhCOCl) reacts with the following reagents.(a) ethanoarrow_forward
- (a) Compound D undergoes a reaction with hydrogen bromide, HBr to produce 2-bromobutane. D exists as cis-trans isomers and decolourises bromine solution in methylene chloride, CH2CI2. (i) Draw and name the structure of compound D. (ii) Draw two (2) constitutional isomers of compound D.arrow_forwardGive reasons for the following :(i) Phenol is more acidic than methanol.(ii) The C—O—H bond angle in alcohols is slightly less than the tetrahedral angle (190°28′).(iii) (CH3)3C—O—CH3 on reaction with HI gives (CH3)3C—I and CH3—OH as the main products and not (CH3)3C—OH and CH3—I.arrow_forward(c) Arrange the following compounds in order of increasing acidity, and explain the reasons fo your choice of order: phenol, cyclohexanol, 2-fluorocyclohexanol, 2-fluoronhenolarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





