
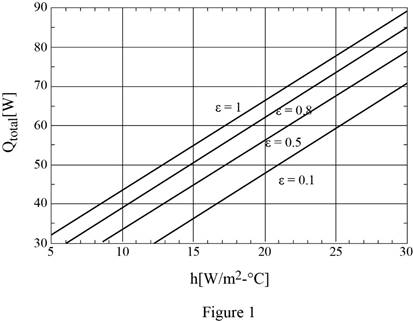
The plotting of rate of heat transfer against the convection heat transfer coefficient for the surface emissivities of 0.1, 0.5, 0.8, and 1. Also, discuss the results.
Answer to Problem 100P
The plotting of rate of heat transfer against the convection heat transfer coefficient for the surface emissivities of 0.1, 0.5, 0.8, and 1 are shown in Figure (1) and results are discussed as below.
Explanation of Solution
Calculate the rate of heat transfer by convection.
Here, change in the temperature is
Calculate the rate of heat transfer by radiation.
Here, surface temperature is
Calculate the total rate of heat transfer from the ball.
Conclusion:
Let us solve for
Substitute
Substitute
Substitute 34.35 W for
Follow the above process to calculate the rate of heat transfer against the convection heat transfer coefficient for the surface emissivities of 0.1 and 0.5 using spreadsheet including equations (I), (II), and (III) as in table (1).
| 5 | 11.4511 | 2.04127 | 13.4924 | 10.2064 | 21.6575 |
| 7.5 | 17.1767 | 2.04127 | 19.2179 | 10.2064 | 27.383 |
| 10 | 22.9022 | 2.04127 | 24.9435 | 10.2064 | 33.1086 |
| 12.5 | 28.6278 | 2.04127 | 30.669 | 10.2064 | 38.8341 |
| 15 | 34.3533 | 2.04127 | 36.3946 | 10.2064 | 44.5597 |
| 17.5 | 40.0789 | 2.04127 | 42.1201 | 10.2064 | 50.2852 |
| 20 | 45.8044 | 2.04127 | 47.8457 | 10.2064 | 56.0108 |
| 22.5 | 51.53 | 2.04127 | 53.5712 | 10.2064 | 61.7363 |
| 25 | 57.2555 | 2.04127 | 59.2968 | 10.2064 | 67.4619 |
| 27.5 | 62.9811 | 2.04127 | 65.0224 | 10.2064 | 73.1874 |
| 30 | 68.7066 | 2.04127 | 70.7479 | 10.2064 | 78.913 |
Continue table (1) for
| 16.3302 | 27.7813 | 20.4127 | 31.8638 |
| 16.3302 | 33.5068 | 20.4127 | 37.5894 |
| 16.3302 | 39.2324 | 20.4127 | 43.3149 |
| 16.3302 | 44.9579 | 20.4127 | 49.0405 |
| 16.3302 | 50.6835 | 20.4127 | 54.766 |
| 16.3302 | 56.4091 | 20.4127 | 60.4916 |
| 16.3302 | 62.1346 | 20.4127 | 66.2172 |
| 16.3302 | 67.8602 | 20.4127 | 71.9427 |
| 16.3302 | 73.5857 | 20.4127 | 77.6683 |
| 16.3302 | 79.3113 | 20.4127 | 83.3938 |
| 16.3302 | 85.0368 | 20.4127 | 89.1194 |
Show the plotting of rate of heat transfer against the convection heat transfer coefficient for the surface emissivities of 0.1, 0.5, 0.8, and 1.0 using Table (1) and (2) as in Figure (1).
Want to see more full solutions like this?
Chapter 2 Solutions
Thermodynamics: An Engineering Approach
- Consider a person standing in a room at 23°C. Determine the total rate of heat transfer from this person if the exposed surface area and the skin temperature of the person arel.7 m2 and 32°C, respectively, and the convection heat transfer coefficient is 5 W/m2· °C. Take the emissivity of the skin and the clothes to be 0.9, and assume the temperature of the inner surfaces of the room to be the same as the air temperature.arrow_forwardA fan forces air over a computer circuit board with an area of 0.01 m2 to keep the circuit board cool. If the temperature of the surface is at 350 K and the incoming air is at 298 K, determine the rate of heat transfer in W. Assume the heat transfer coefficient is 20 W/(m2K). Report your answer to one decimal place.arrow_forward2-105 The outer surface of a spacecraft in space has an emis- sivity of 0.6 and an absorptivity of 0.2 for solar radiation. If solar radiation is incident on the spacecraft at a rate of 1000 W/m², determine the surface temperature of the spacecraft when the radiation emitted equals the solar energy absorbed.arrow_forward
- Determine the rate of heat loss from the pipe by natural convection, in kW. 18. An aluminum pan whose thermal conductivity is 237 W/m · °C has a flat bottom whose diameter is 20 cm and thickness 0.4 cm. Heat is transferred steadily to boiling water in the pan through its bottom at a rate of 500 W. If the inner surface of the bottom of the pan is 105°C, determine the temperature of the outer surface of the bottom of the pan.arrow_forwardThe inner and outer glasses of a 2-m × 2-m double pane window are at 18°C and 6°C, respectively. If the 1-cm space between the two glasses is filled with still air, determine the rate of heat transfer through the air layer by conduction, in kW.arrow_forwardThe inner and outer surfaces of a 5-m x 6-m brick wallof thickness 30 cm and thermal conductivity 0.69 W/m · °C aremaintained at temperatures of 20°C and 5°C, respectively.Determine the rate of heat transfer through the wall, in Warrow_forward
- Hot air at 80°C is blown over a 2-m x 4-m flat surface at 30°C. If the convection heat transfer coefficient is 90 W/m2.°C, determine the rate of heat transfer from the air to the plate, in kW. The rate of heat transfer from the air to the plate is kW.arrow_forwardA room is initially at the outdoor temperature of 25°C. Now a large fan that consumes 200 W of electricity when running is turned on (Fig. 2–51). The heat transfer rate between the room and the outdoor air is given as Q · = UA(Ti − To) where U = 6 W/m2 ·°C is the overall heat transfer coefficient, A = 30 m2 is the exposed surface area of the room, and Ti and To are the indoor and outdoor air temperatures, respectively. Determine the indoor air temperature when steady operating conditions are established.arrow_forwardA ball with diameter of 4 inches, is suspended in the middle of a room at 530°R. The ball's surface is maintained at a temperature of 700°R If the convection heat transfer coefficient is 0.04 kW/m2-°C and the emissivity of the surface is 0.7, determine the total rate of heat transfer from the ball.arrow_forward
- A mother takes the milk to be given to her baby from the refrigerator and pours it into a cylindrical bottle for heating. The height of the milk in the bottle is 10 cm. Then he places the bottle into a large container with hot water at a temperature of 62 ° C. The heat transfer coefficient between hot water and bottle is 130 W / m2.oC. The process of heating the milk from 4 0C to 40 0C is required to be completed within 6 minutes. What should be the diameter of the bottle used? The properties of milk can be equal to the properties of water at the same temperature.arrow_forwardA cat has a body temperature of 48 ºC. She stays inside a closed air conditioned room that has an inside wall temperature and inside air temperature of 40 and 35 ºC, respectively. The temperature of the room’s outside wall and the air outside the room are measured to be 45 and 50 ºC, respectively. The convection heat transfer coefficient between the cat and the inside air is 5 W/m2 ºC and the convection heat transfer coefficient between the outside air and outside wall is 35 W/m2 ºC. Use 0 ºC = 273.15 K to calculate the heat losses per unit area (W/m2 ) from the cat if the emissivity of the cat is 0.85.arrow_forwardDetermine the steady rate of heat transfer (in W) through the glass window. The room is maintained at 24°C while the temperature of the outdoors is –5°C.arrow_forward
 Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning
Principles of Heat Transfer (Activate Learning wi...Mechanical EngineeringISBN:9781305387102Author:Kreith, Frank; Manglik, Raj M.Publisher:Cengage Learning
