
Concept explainers
(a)
Interpretation:
In
Concept introduction:
The molecular orbital is a combination of two atomic orbitals. It is used to represent the regions in a molecule where the electron is likely to be present in an orbital. It represents the wave-like nature of an electron in a molecule. It may be symmetric or antisymmetric. It may be bonding, antibonding or non-bonding. It may be HOMO or LUMO.
Answer to Problem 28.4P
In
Explanation of Solution
The structure of
This molecule contains seven carbon atoms. In case of the odd number of carbon atoms chain which is in conjugation, the molecular orbitals do not separate out into two equal halves in bonding and antibonding molecular orbitals. Along with that it gives rise to one molecular orbital whose energy is equal to that of
In
(b)
Interpretation:
Each molecular orbital of
Concept introduction:
The molecular orbital is a combination of two atomic orbitals. It is used to represent the regions in a molecule where the electron is likely to be present in an orbital. It represents the wave-like nature of an electron in a molecule. It may be symmetric or antisymmetric. It may be bonding, antibonding or non-bonding. It may be HOMO or LUMO.
Answer to Problem 28.4P
The molecular orbitals
Explanation of Solution
In molecular orbital theory, the MO is said to be symmetric or anti-symmetric depending on the relative phase of the two terminal carbons. In symmetric MO, the peaks reflect across the reference plane into the peaks and troughs reflect into troughs. On the other hand, in antisymmetric MO, the peaks reflect into troughs and vice versa. According to the general principle, the even number molecular orbitals are antisymmetric and odd number molecular orbitals are symmetric. Therefore, the molecular orbitals
The molecular orbitals
(c)
Interpretation:
The carbon on which the positive charge is delocalized is to be stated. The explanation on the basis of resonance structures and molecular orbital arguments is to be stated.
Concept introduction:
The molecular orbital is a combination of two atomic orbitals. It is used to represent the regions in a molecule where the electron is likely to be present in an orbital. It represents the wave-like nature of an electron in a molecule. Most of the organic structures cannot be represented using a single Lewis structure. Therefore, there exists more than one Lewis structure for representing a molecule or ion. These structures are known as resonance structures. The delocalization of electrons results in the formation of resonance structure.
Answer to Problem 28.4P
The positive charge is delocalized over
Explanation of Solution
The resonance structure of
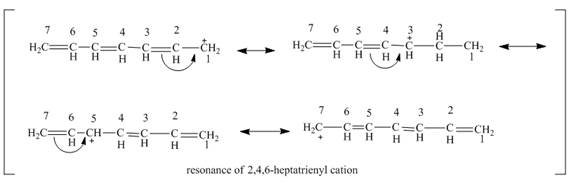
Figure 1
The resonance structures of
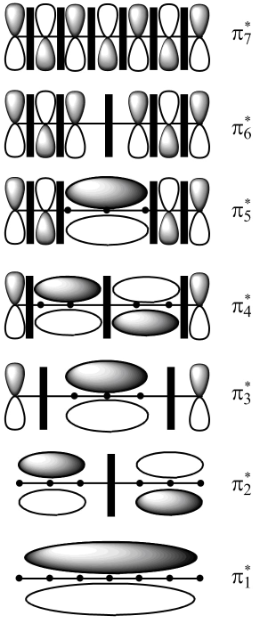
Figure 2
According to the general principle if the node is passing through the carbon then the positive charge is not present on that carbon. In this molecular orbital diagram, the node is passing through the
The positive charge is delocalized over
Want to see more full solutions like this?
Chapter 28 Solutions
Organic Chemistry
- There are two C–C single bonds in penta-1,3-diyne. (a) Which of those bonds would you expect to be stronger? (b) Which of those bonds would you expect to be shorter? (c) The molecule is moderately polar, with a dipole moment of 1.37 D. In which direction would you expect the dipole moment to point? Explain. Penta-1,3-diynearrow_forwardSodium triacetoxyborohydride, NaBH(OAc)3, is a mild reducing agent that reduces aldehydesmuch more quickly than ketones. It can be used to reduce aldehydes in the presence of ketones,such as in the following reaction:CH3 C CH2O OC H CH3 C CH2OCH2OHNaBH(OAc)3CH3COOH(a) Draw a complete Lewis structure for sodium triacetoxyborohydride.(b) Propose a mechanism for the reduction of an aldehyde by sodium triacetoxyborohydridearrow_forward'' Which of the statements A-E is NOT correct about azidothymidine (AZT), an antiviral drug? (a) The molecule contains a sigma bond formed by the overlap of a nitrogen sp orbital with a nitrogen sp2 H3C. NH orbital (b) It contains a secondary (2°) amine (c) It contains a primary (1°) alcohol (d) The molecule contains the following functional groups: alkene, amide, ether (e) It contains one atom with a +1 formal charge and one with a -1 formal charge HOH2C N=N=Narrow_forward
- 44) The valance shell of C-atom has 2s²2p². How can this configuration leads to a tetravalent C- atom?arrow_forwardPyrethrins, such as jasmolin II(below), are a group of naturalcompounds synthesized by flowers of the genus Chrysanthemum(known as pyrethrum flowers) to act as insecticides.(a) Circle and name the functional groups in jasmolin II.(b) What is the hybridization of the numbered carbons?(c) Which, if any, of the numbered carbons are chiral centers?arrow_forwardExplain how covalent bonds are formed in each of the following compounds in terms of orbital hybridisation and overlap of orbitals (i) Ethene, C2H4 (ii) Ethyne, C2H2arrow_forward
- The pentadienyl radical, H2C“CH¬CH“CH¬CH2#, has its unpaired electron delocalized over three carbon atoms.(a) Use resonance forms to show which three carbon atoms bear the unpaired electron.(b) How many MOs are there in the molecular orbital picture of the pentadienyl radical?(c) How many nodes are there in the lowest-energy MO of the pentadienyl system? How many in the highest-energy MO?(d) Draw the MOs of the pentadienyl system in order of increasing energyarrow_forwardThe pentadienyl radical, H2C“CH¬CH“CH¬CH2#, has its unpaired electron delocalized over three carbon atoms.(a) Use resonance forms to show which three carbon atoms bear the unpaired electron.(b) How many MOs are there in the molecular orbital picture of the pentadienyl radical?(c) How many nodes are there in the lowest-energy MO of the pentadienyl system? How many in the highest-energy MO?(d) Draw the MOs of the pentadienyl system in order of increasing energy. (continued)762 CHAPTER 15 Conjugated Systems, Orbital Symmetry, and Ultraviolet Spectroscopy(e) Show how many electrons are in each MO for the pentadienyl radical (ground state).(f) Show how your molecular orbital picture agrees with the resonance picture showing delocalization of the unpairedelectron onto three carbon atoms.(g) Remove the highest-energy electron from the pentadienyl radical to give the pentadienyl cation. Which carbon atomsshare the positive charge? Does this picture agree with the resonance picture?(h) Add an…arrow_forwardJ of Br G K OH Br H L OCH 3 of 1 M OH NH (i) Compound H is an example of what functional group? Select alternative (ii) Compound G is classified as a: Select alternative ✓. (iii) Which compound has all carbons sp2 hybridised? Select alternative ✓ (iv) Which two compounds in Table 1 are constitutional isomers? Select alternative (v) Which statement best describes the stereochemistry of compo G? Select alternative (vi) Which compound in Table 1 is the MOST polar? Select alternative (vii) What is the systematic (IUPAC) name of compound G? Select alternative (viii) Which compounds will have MORE than 4 signals in their 13C NMR spectra? Select alternative (ix) Which electrophile from Table 1 will react fastest in an SN1 reaction? Select alternative (x) How many constitutionally isomeric alkenes will be formed when compound L reacts with NaOH? Select alternative (xi) Which compound(s) from Table 1 can be used to form a Grignard reagent? Select alternative (xii) Which functional groups can be…arrow_forward
- Your chemistry professor draws a number of molecules on the board: (1) CH4 ; (2) H2C=CH2 ; (3) H2C=C=CH2 ; and (4) H2C=C=C=CH2. You muse about all the molecules that chemists draw on a two-dimensional board and wonder which ones are actually planar, existing basically as they appear on the board, and which ones are not plane but rather three-dimensional. Answer ALL of the following questions. What are the specific orbital overlaps (i.e., sp3-sp3) that are in each of the molecules? What are the bond angles for each central atom in each molecule? Which molecules are planar and which are non-planar?arrow_forwardYour chemistry professor draws a number of molecules on the board: (1) CH4 ; (2) H2C=CH2 ; (3) H2C=C=CH2 ; and (4) H2C=C=C=CH2. You muse about all the molecules that chemists draw on a two-dimensional board and wonder which ones are actually planar, existing basically as they appear on the board, and which ones are not plane but rather three-dimensional. Answer the following questions. What is the geometry and hybridization of the carbon in CH4? What is the geometry and hybridization of each central carbon atom in the remaining molecules? Draw each molecule showing the bonds and identify each bond in all the molecules as s or p. What are the specific orbital overlaps (i.e., sp3-sp3) that are in each of the molecules? What are the bond angles for each central atom in each molecule? Which molecules are planar and which are non-planar?arrow_forwardAssign the configuration Z or E to each double bond, where appropriate, in the following molecules. (a) (b) (c) Br Br NO2arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





