
Draw the four water molecules that can hydrogen-bond to this water molecule. Label the bonds and the partial negative and positive charges that account for the formation of these hydrogen bonds.
To draw: The four water molecules that can form hydrogen-bond to the given water molecule and label the bonds and the partial negative and positive charges that account for the formation of these hydrogen bonds.
Introduction: A water molecule is shaped like a ‘V’ that consists of an oxygen atom and two hydrogen atoms. Oxygen is more electronegative than hydrogen. Thus, the shared electrons in the O-H covalent bond are more attracted by the oxygen atom.
Answer to Problem 1IQ
Pictorial representation: Fig.1 represents the four water molecules that can form hydrogen-bond for the given water molecule and label the bonds and the partial negative and positive charges that account for the formation of these hydrogen bonds.
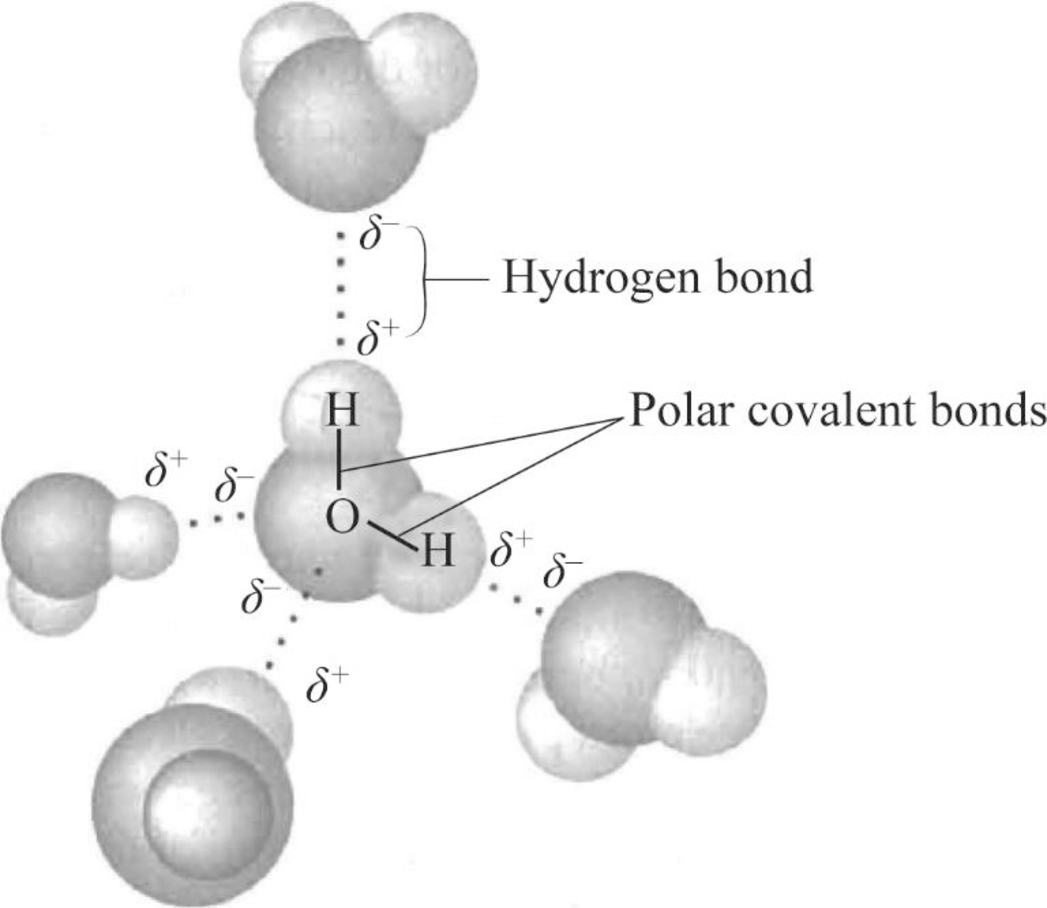
Fig.1: Four water molecules bind with one water molecule
Explanation of Solution
Electronegativity is the tendency of an atom in a molecule to attract electrons shared in a covalent bond. If one atom is more electronegative, the electrons would be more on the side of that atom. Oxygen is more electronegative than hydrogen. In a water molecule, there are two polar covalent bonds between oxygen and hydrogen. Hence, there are two regions of partial negative charge on oxygen and a partial positive charge on each hydrogen atom.
The partial negatively charged oxygen atom of a water molecule is attracted to the partial positively charged hydrogen of the other water molecule. As per Fig.1, atoms on the partial charged regions can bind to oppositely charged regions on other adjoining water molecules. There are four regions of partial charge in a water molecule. Thus, the central water molecule can form a hydrogen bonds with four other water molecules.
Want to see more full solutions like this?
Chapter 3 Solutions
Study Guide for Campbell Biology
- :0-H-1--:N-H Hydrogen Bond Which statement best helps explain the formation of the hydrogen bond represented in the figure? A The oxygen has a partial positive charge, and the nitrogen has a partial negative charge. (B) The nitrogen has a partial negative charge, and the hydrogen attached to the oxygen has a partial positive charge.arrow_forwardGive examples of four different types of bond involving hydrogen atoms. (polar covalent, non-polar covalent, hydrogen bonding and ionic). Comment on any unusual features and consequences of the examples you cite.arrow_forwardRefer to figure 2.15 to draw a picture of on NaCl molecule interacting with several water molecules. Label all molecules and ions appropriately and, as usual, use dotted lines to represent any hydrogen bonds.arrow_forward
- Draw the dipeptide that results when a peptide bond is formed between the two glycine molecules shown here. Draw it as it would occur at the pH of most body fluids. Include all hydrogen atoms.arrow_forwardPolar covalent bonds can be found in water molecules. Define or describe this type of bond.arrow_forwardIndicate whether the statement below is true or false.Any covalently bonded H atom can participate in a hydrogen bond if it comes in close proximity with an oxygen atom that forms part of a water molecule.arrow_forward
- 90% of our body is made of water. Use a water molecule to describe the relationship among all the following polar molecules, intramolecular and intermolecular forces, hydrophilic and hydrophobic interactions.arrow_forwardAmino acids are the building units of proteins. These compounds contain at least one amino group and a carboxyl group. Glycine exists in three possible types, depending on the pH of the solution. Fully protonated: Dipolar ion: Completely ionized: pH 1.0; Predict which structure of glycine might be dominant at pH 7.0 and pH 12.0. The pKa value of the carboxyl group is 2.3 and the ammonium group is 9.6.Please explain.arrow_forwarddraw the hydrogen bonds for the following nucleic acid base pair: G and Carrow_forward
- The “octet rule” in chemistry helps predict the types of bonds thatatoms will form. In general, an atom will be most stable if it fills itsouter shell of 8 electrons. Atoms with fewer than 4 valence electronstend to donate electrons and those with more than 4 valence electronstend to accept additional electrons; those with exactly 4 can do both.Using this rule, determine what category each of the followingelements falls into: N, S, C, P, O, H, Ca, Fe, and Mg. (You will needto work out the valence of the atoms.)arrow_forwardType of Bond: Choices: H-bond Electrostatic Interaction Hydrophobic bond Disulfide bond Peptide bond Level of Protein structure: Choices: Primary Secondary Tertiary Quaternary Method/s of denaturation. CHECK ALL THAT APPLY Heating to 100 degrees Celsius Addition of nitric acid Reaching Isoelectric point Addition of mercuric chloride Addition of sulphosalicylic acid Addition of alcohol Addition of ammonium sulfatearrow_forwardIdentify the IMF that would form between the side chains of: Asn and Thr Hydrogen bonds Hydrophobic interactions Disulfide bonds lon-dipole Salt bridgesarrow_forward
 Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning
Biology (MindTap Course List)BiologyISBN:9781337392938Author:Eldra Solomon, Charles Martin, Diana W. Martin, Linda R. BergPublisher:Cengage Learning Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning
Human Biology (MindTap Course List)BiologyISBN:9781305112100Author:Cecie Starr, Beverly McMillanPublisher:Cengage Learning


