
Concept explainers
- a. Draw three constitutional isomers with molecular formula C3H8O.
- b. How many constitutional isomers can you draw for C4H10O?
(a)
Interpretation:
The three constitutional isomers of molecular formula
Concept introduction:
Constitutional isomers are having the same molecular formula and different bond connectivity between the atoms.
The general formula for an alkane is
Equation for finding constitutional isomers for a molecule
Answer to Problem 1P
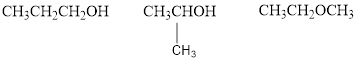
Explanation of Solution
The given molecular formula is
Therefore,
The two possible alcohols are,
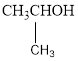
The possible ether is,
(b)
Interpretation:
The numbers of constitutional isomers of molecular formula
Concept introduction:
Constitutional isomers are having the same molecular formula and different bond connectivity between the atoms.
Answer to Problem 1P
The numbers of constitutional isomers of molecular formula
Explanation of Solution
The given molecular formula is
Therefore,
There will be five alcohol isomers (due to four carbons) and two ether isomers.
Hence,
Want to see more full solutions like this?
Chapter 4 Solutions
Essential Organic Chemistry (3rd Edition)
Additional Science Textbook Solutions
Chemistry
Inorganic Chemistry
Introductory Chemistry (5th Edition) (Standalone Book)
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
- XX. CH₂ H. H Label each pair of compounds below as: تے H₂C a. conformational isomers b. configurational isomers constitutional isomers identical not isomers C. d. e. CH3 CH 3 CH 3 and CI H CH3 and CH3 H H₂C- Н. H H H3C12 CH₂ CH3 CH3 CH₂ CI H3C- * The same letter can be use more than once!arrow_forward9) There are 3 different cyclopropane molecules with the formula GHĄC12. a. Draw and build the 3 molecules. b. Below each drawing, name each molecule with correct nomenclature. Label a pair that are constitutional isomers. d. Label a pair that are stereoisomers (or configurational isomers). С.arrow_forward2. Draw CH3-CH=C(CH3)Br first as the cis isomer, then as the trans isomer:arrow_forward
- 3. Geometric isomers of the following compounds and give the IUPAC name: a. CI-CH = C- CH3 b. CH3CH2CH=CHCH=CH2 CH3 4. possible equatorial axial relationships for the cis and trans for the compound CH2CH3 CH Which is the conformer? Explain (in not more than two lines in your own words)arrow_forwardDraw two constitutional isomers that share the molecular formula C2H,N. Your structures will have the same molecular formula but will have different connectivities.arrow_forwardCH3-CH2-CH-CH3 CH3-CH-CH2-OH CH2-CH2-OH CH2-CH-OH CH3-CH2-CH2-CH2-OH CH3-CH2-O-CH2-CH3 ОН CH3 CH2.CH3 CH3 II II IV V VI I and Il are A. conformations (or conformational isomers) B. chain (skeletal) isomers C. functional isomers 31. D. positional isomers E. not isomersarrow_forward
- Consider 1,2-dimethylcyclohexane.a. Draw structures for the cis and trans isomers using a hexagon for the six-membered ring.b. Draw the two possible chair conformations for the cis isomer. Which conformation, if either, is more stable?c. Draw the two possible chair conformations for the trans isomer. Which conformation, if either, is more stable?d. Which isomer, cis or trans, is more stable and why?arrow_forwardDraw at least four isomers with molecular formula given below. C4H10Oarrow_forwardAnalyze the two Newman projections and determine the relationship between the two. How would you describe the relation between conformations when they are maintained at a temperature too low to permit them to interconvert? CH3 CH3 Br. H H H H A. Identify the relationship. They are identical. They are structural isomers. They are stereoisomers. They are conformers. H H -I H Br H B. What is the relationship at low temperatures? They are identical. They are conformational diastereomers. They are structural isomers. They are conformational enantiomers.arrow_forward
- 3. Draw and name all of the constitutional isomers of C4H7Br3.arrow_forwardHow many isomers can be drawn for C₆H₁₄? A.) 3 B.) 5 C.) 4 D.) 1 E.) 2arrow_forwardQ6.7 B and D CH3 „CH3 H3C, CH3 CH3 CH3 H;C H3C" CH3 CH3 CH3 H;C B D F What is the relationship between molecules B and D? O They are the same molecule O They are enantiomers of one another O They are diastereomers of one another O They are constitutional isomers O There's no relationship between them, they have completely different chemical formulas Q6.8 B and E CH3 CH3 H3C,, CH3 CH3 H3C" CH3 CH; CH3 H;C CH3 D. E F What is the relationship between molecules B and E? O They are the same molecule O They are enantiomers of one another O They are diastereomers of one another O They are constitutional isomers O There's no relationship between them, they have completely different chemical formulasarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





