
Concept explainers
Interpretation: The product formed after the crossed-aldol condensation between 2-nitrobenzaldehyde and acetone in the first step for synthesis of indigo needs to be determined.
Concept Introduction:In crossed-aldol condensation, two different carbonyl compounds (both with alpha hydrogen atom) undergo condensation reaction together. There is a possibility of 4 products in such type of condensation reaction.
Explanation of Solution
The formation of indigo is represented as follows:
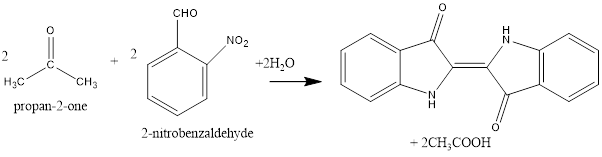
The first step of the reaction is crossed aldol condensation. The given reactants are 2-nitrobenzaldehyde and acetone or propan-2-one. Both of the reactantshasa carbonyl group thus, both are carbonyl compounds. Since, both the carbonyl compounds are different thus, crossed aldol condensation is possible.
The structure of reactants is represented as follows:
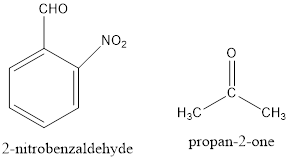
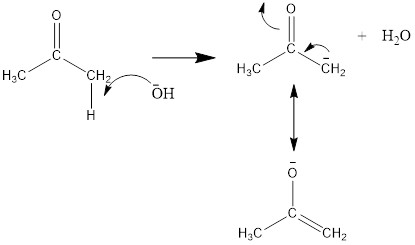
Here, the removal of alpha hydrogen from 2-nitrobenzaldehyde is not possible because the negative charge on the double bond is not stable. Thus, the hydroxide ion of water removes the alpha hydrogen of acetone or propan-2-one.
The mechanism for the crossed aldol condensation will be as follows:
Now, the negative charge will attack the carbonyl group of 2-nitrobenzaldehyde as follows and results in the formation of a hydroxy
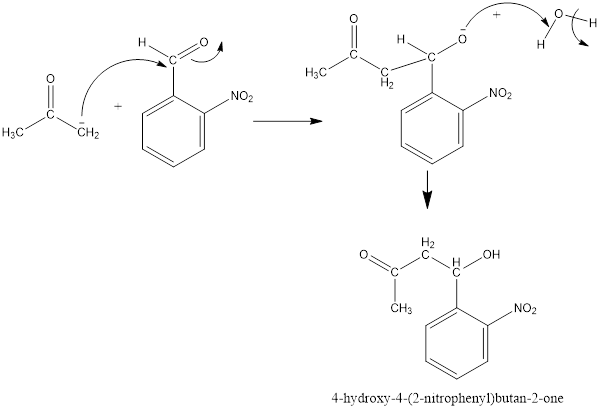
Thus, the product formed in the first step will be as follows:
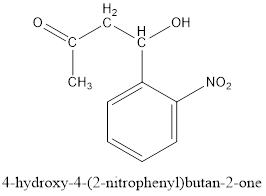
After this series of condensations, tautomerizations, a retro Claisen condensation and elimination reactions result in the formation of indigo.
Want to see more full solutions like this?
Chapter 45 Solutions
EBK A SMALL SCALE APPROACH TO ORGANIC L
- Give the reagents that should be used to carry out the following synthesisarrow_forward-Hydroxyketones and -hydroxyaldehydes are also oxidized by treatment with periodic acid. It is not the -hydroxyketone or aldehyde, however, that undergoes reaction with periodic acid, but the hydrate formed by addition of water to the carbonyl group of the -hydroxyketone or aldehyde. Write a mechanism for the oxidation of this -hydroxyaldehyde by HIO4.arrow_forwardWhat is the product resulted due to the reaction of 2-hydroxy-3-methoxybenzaldehyde with ethyl bromoacetate in a basic solution indicating all the reagents and intermediates occurred during the reaction? What is the product obtained when product A reacted with ethyl acetate in the same basic solution?arrow_forward
- Draw the structure of the hydroxyaldehyde product from the self-aldol reaction of each of the following aldehydes: (a) propanal; (b) phenylethanal; (c) 3-phenylpropanal; (d) benzaldehyde.arrow_forwardExplain how ethyl-2-phenyl acetate could be formed under the reaction conditions used. 2-Phenylacetic acid + 3-methyl-1-butanol + Ethanol + H2SO4 made ethyl-2-phenyl acetate Acetic acid + 3-methyl-1-butanol + Ethanol + H2SO4 made ethyl-2-phenyl acetatearrow_forwardcomplete the following synthesesarrow_forward
- A synthetic organic molecule, G, which contains both aldehyde and ether functional groups, is subjected to a series of reactions in a multi-step synthesis pathway. In the first step, G undergoes a Wittig reaction, leading to the formation of an alkene, H. Subsequently, H is treated with an ozone (O3) reagent followed by a reducing agent in an ozonolysis reaction, resulting in the formation of two different products, I and J. Considering the functional groups present in G and the nature of the reactions involved, what are the most probable structures or functional groups present in products I and J? A. I contains a carboxylic acid group, and J contains an aldehyde group. B. I contains a ketone group, and J contains an alcohol group. C. I and J both contain aldehyde groups. D. I contains an ester group, and J contains a ketone group. Don't use chat gpt.arrow_forward2-Ethyl-1-hexanol was needed for the synthesis of the sunscreen octyl p-methylcinnamate. Show how this alcohol could be synthesized (a) by an aldol condensation of butanal and (b) by a malonic ester synthesis starting with diethyl malonate.arrow_forward2 H3C H3C H C→XT OH H3C The aldol reaction is a carbonyl condensation reaction between two carbonyl partners and involves a combination of nucleophilic addition and a-substitution steps. One partner is converted into an enolate ion nucleophile and adds to the electrophilic carbonyl group of the second partner. In the classic aldol reaction, the carbonyl partners are aldehydes or ketones, although aldehydes are more reactive. The product is a ß-hydroxy carbonyl compound. base :0: OH H H Under reaction conditions slightly more vigorous than those employed for the aldol reaction, the ß-hydroxyl group is eliminated in an E1cB dehydration to give an a,ß-unsaturated carbonyl compound. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instruct ns H3C heat OH H3C :0: H + H₂O Harrow_forward
- Identify the best reagents to complete the following reaction. HO, CIarrow_forwardWhat reaction converts benzoic acid to m-bromobenzoic acid? Alkylation Acylation Halogenation Hydrohalogenation Hydration Reduction Oxidation Nitration Sulfonationarrow_forwardDraw out the reaction mechanism for cyclohexanol to cyclohexanone. Sodium hypochlorite oxidation of an alcohol to a ketone with the product being cyclohexanone.arrow_forward
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


