
Concept explainers
Interpretation: The reason due to which the products in the cracking of dicyclopentadieneare distilled very slowly needs to be explained.
Concept Introduction:
Dicyclopentadiene has chemical formula C10H12. It is clear yellow color liquid with acrid odor. It is used in resins and also used in adhesives, pants and inks.
It is abbreviated as DCPD and the structure is represented as follows:
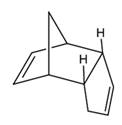
Answer to Problem 1Q
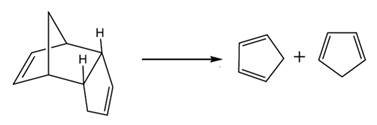
The products are heated slowly to avoid the formation of dicyclopentadiene again.
Explanation of Solution
In the cracking of dicyclopentadiene, the products formed are as follows:
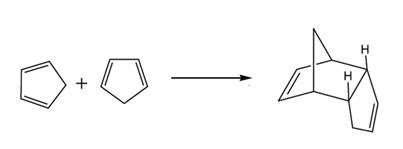
If the products heated strongly, both the products react to form the cyclic reactant again and following reaction take place.
Or,
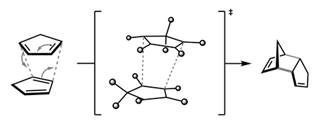
Thus, to avoid the reverse reaction, products are heated slowly.
Want to see more full solutions like this?
Chapter 48 Solutions
Macroscale and Microscale Organic Experiments
- List three reason why xylene is often used in Diels-Alder reactions.arrow_forwardWhat is the function of CH2Cl2 in the bromination reactions? Why can it fulfil this rolearrow_forwardIf the following molecules undergo free radical substitution, what is the order in which the hydrogen is easily replaced by bromine?arrow_forward
- What would be the major product obtained from the reaction of Br2 with 1-butene if the reaction were carried out in the solvent dichloromethane? Draw the molecule.arrow_forwardNucleophilic substitution is a general reaction of organic compounds. Why, then, are alkyl halides the most common substrates, and halide anions the most common leaving groups?arrow_forwardWrite the reaction of bromine addition to an alkene on an example and the mechanism.arrow_forward
- ORGANIC CHEMISTRY - PERICYCLIC REACTIONS Draw the mechanism, explain the stereochemistry of the products, indicate the type of reaction.arrow_forwardWhy is the free radical reaction not the most preferred way to prepare alkyl halidesarrow_forwardAnalyze the different substitution products formed in case of furan when it is reacted with iodine. Write the reaction mechanism involved when furan reacted with iodine and evaluate the formation of products.arrow_forward
- Does Markovnikov's rule apply to the addition of HX to vinyl halides ?arrow_forwardhow many structurally isomeric products form in the free radical bromination of hexane?arrow_forwardThe aerobic oxidation of para-xylene to terephthalic acid is an important process in industrial chemistry. Discuss why the oxidation of the second methyl group requires harsher conditions than the oxidation of the first methyl group. You should accurately reference all your bibliographic material.arrow_forward
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole

