
Concept explainers
MATHEMATICAL The hydrolysis of a phenylalanine-containing peptide is catalyzed by
Interpretation:
The values of
Concept introduction:
In an enzymatic reaction,
The
Answer to Problem 29RE
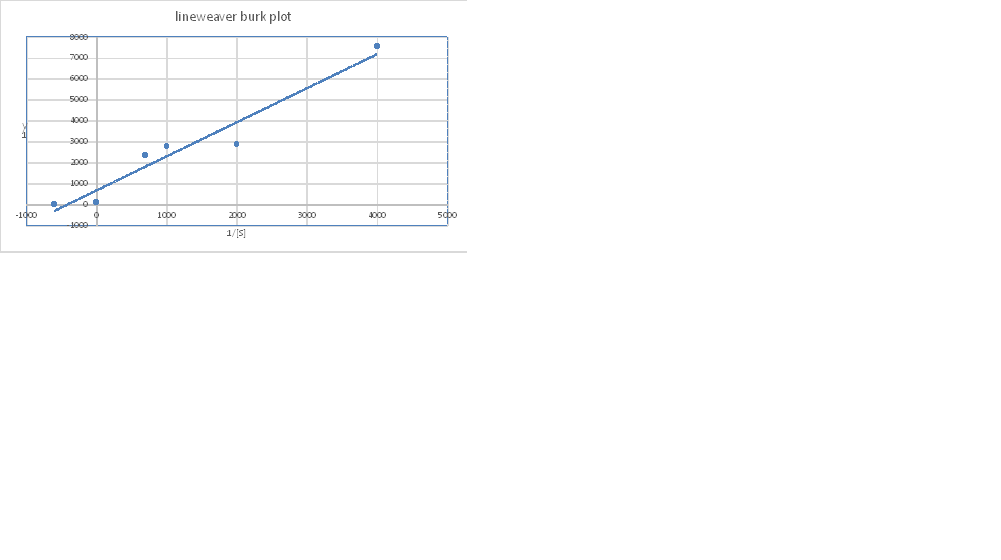
The graph shows a straight line and on extrapolating the line, it intersects X-axis as well as Y-axis, and the desired value is determined.
The value of the Y-intercept is 450, which also represents
So, the value of
Conversion of
The value of the X- intercept is -800, which represents the value of
So, the value of
Explanation of Solution
Given information:
The reciprocal of the substrate concentration and the velocity is required to draw the respective plots. Before plotting the graph, the data must be converted. The reaction velocity data is given in mole/min. It needs to be converted into mole/sec by dividing by 60.
In enzyme kinetics, the values of
This equation is further modified so that it can represent in a straight line.
Modification of the equation is,
From this equation, the values of both
With the help of the Lineweaver–Burk plot, the values of both
Want to see more full solutions like this?
Chapter 6 Solutions
Biochemistry
- MATHEMATICAL Determine the values of KM and Vmax for the decarboxylation of a -keto acid given the following data. substrateconcentration(molL1)Velocity(mMmin1)2.5001.0000.7140.5260.2500.5880.5000.4170.3700.256arrow_forwardMATHEMATICAL Consider the reaction AB+C, where G=0.00. (a) What is the value of G (not G) when the initial concentrations of A, B, and C are 1 M, 103M,and106M? (b) Try the same calculations for the reaction D+EF, for the same relative order of concentrations. (c) Try the same calculations for the reaction GH, if the concentrations are 1Mand103M for G and H, respectively.arrow_forwardMATHEMATICAL For the Vmax obtained in Question 26, calculate the turnover number (catalytic rate constant) assuming that 131024mol of enzyme were used.arrow_forward
- MATHEMATICAL What is the ratio of concentrations of acetate ion and undissociated acetic acid in a solution that has a pH of 5.12?arrow_forwardMATHEMATICAL If a reaction can be written AB, and the G is 20kJmol1, what would the substrate/product ratio have to be for the reaction to be thermodynamically favorable?arrow_forwardMATHEMATICAL Calculate the ATP yield for the complete oxidation of one molecule of palmitic acid (16 carbons). How does this figure differ from that obtained for stearic acid (18 carbons)?arrow_forward
- MATHEMATICAL Using the information in Table 20.2, calculate G for the following reaction: 2Cytaa3[oxidized;Fe(III)]+2Cytb[reduced;Fe(II)]2Cytaa3[reduced;Fe(II)]+2Cytb[oxidized;Fe(III)]arrow_forwardREFLECT AND APPLY Draw Haworth projection formulas for dimers of glucose with the following types of glycosidic linkages: (a) A (14) linkage (both molecules of glucose in the form) (b) An ,(11) linkage (c) A (16) linkage (both molecules of glucose in the form)arrow_forwardMATHEMATICAL What is the ratio of HEPES/HEPES-H+ in a HEPES buffer at pH 7.9?arrow_forward
- REFLECT AND APPLY A researcher claims to have discovered a variant form of glycogen. The variation is that it has very few branches (every 50 glucose residues or so) and that the branches are only three residues long. Is it likely that this discovery will be confirmed by later work?arrow_forwardMATHEMATICAL Predict the predominant ionized forms of the following amino acids at pH 7: glutamic acid, leucine, threonine, histidine, and arginine.arrow_forwardREFLECT AND APPLY An amino acid mixture consisting of lysine, leucine, and glutamic acid is to be separated by ion-exchange chromatography, using a cation-exchange resin at pH 3.5, with the eluting buffer at the same pH. Which of these amino acids will be eluted from the column first? Will any other treatment be needed to elute one of these amino acids from the column?arrow_forward
 BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
BiochemistryBiochemistryISBN:9781305961135Author:Mary K. Campbell, Shawn O. Farrell, Owen M. McDougalPublisher:Cengage Learning
