
Concept explainers
(a)
INTREPRETATION:
The product formed for the reaction between fumarate and
CONCEPT INTRODUCTION:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Nucleophilic nature depends on the negative charge present in the molecule, the solvent in which it present and the electronegativity of the atom.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
The curved arrows are generally used to indicate the flow of electrons present in the reaction.
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
In addition reaction of
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
Oxidizing Reagents: The chemical agents used to add oxygen or remove hydrogen which finally reduced on oxidizing the other compound.
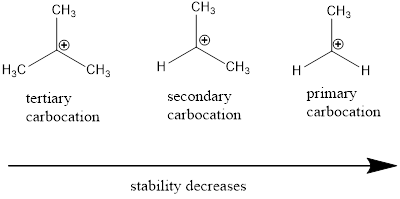
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:
Enantiomers: they are chiral molecules whose mirror images are not superimposable.
R and S nomenclature: it is used to assign the molecule using CIP rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
(b)
INTREPRETATION:
The product formed for the reaction between maleate and
CONCEPT INTRODUCTION:
Nucleophile: Nucleophiles are electron rich compounds which donates electrons to electrophilic compounds which results in bond formation.
Nucleophilic nature depends on the negative charge present in the molecule, the solvent in which it present and the electronegativity of the atom.
Electrophile: Electrophiles are electron deficient compounds which accepts electrons from nucleophiles that results in bond formation.
Chemical reaction involves bond making and breaking of two or more reactants in order to attain products from the reactants.
The curved arrows are generally used to indicate the flow of electrons present in the reaction.
Addition Reaction: It is defined as chemical reaction in which two given molecules combines and forms product. The types of addition reactions are electrophilic addition, nucleophilic addition, free radical additions and cycloadditions. Generally, compounds with carbon-hetero atom bonds favors addition reaction.
In addition reaction of alkenes when two substituents are placed on same side of
Oxidation Reaction: It involves loss of electrons, addition of oxygen atoms or removal of hydrogen atoms.
Oxidizing Reagents: The chemical agents used to add oxygen or remove hydrogen which finally reduced on oxidizing the other compound.
Carbocation: it is carbon ion that bears a positive charge on it.
Carbocation stability order:

Enantiomers: they are chiral molecules whose mirror images are not superimposable.
R and S nomenclature: it is used to assign the molecule using CIP rules.
The CIP rules are as follows:
Select the chiral carbon and assign the numbers according to the decreasing atomic mass of atoms attached to it.
If the numbering follows clockwise direction then the molecule is termed as R and if it follows anti-clockwise direction then molecule is termed as S.
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
Essential Organic Chemistry (3rd Edition)
- 1. The enzyme that catalyzes the reversible reaction below requires what cofactor? A. Pyridoxal phosphate B. Paradoxal phosphate C. Tetrahydrofolate D. S-Adenosyl methionine 2. Skeletal muscle exports excess nitrogen to the liver in the form of... A. Alanine B. Tyrosine C. Pyruvate D. Glycine 3. In the urea cycle, ornithine transcarbamoylase condenses a ornithine with what molecule to produce citrulline? A. Carbamate B. Aspartate C. Phosphate D. Carbamoyl phosphatearrow_forwardWhat percent of a cell’s overall mass is accounted for byproteins?arrow_forward5. Match the following: A. A triglyceride on hydrolysis gives, 1. Sphingosine Fatty +Carbohydrate 2. A non-saponifiable lipid B. A wax consists of, C. Phosphoglyceride consists of, 3. Sphingosine Fatty acid + Phosphoric acid+ Carbohydrate 4. A set of 3 6-membered And a 5-membered ring Fused together. 5. Saponifiable lipid. D. A sphingomyelin consists of, E. A glycolipid consists of, F. A steroid is a special type of ring system Consists of 6. Fatty acids and long Chain alcohols 7. Glycerol, Fatty acids+ Phosphoric acids G. A prostaglandin is an example of, H. Triglycerides, phospholipids and glycol Lipids are examples of 8. Fatty acids + Glycerolarrow_forward
- 13. Which of the following(s) is(are) not "ketone bodies"? B. Acetoacetate C. Acetyl-CoA A. Acetone D. D-B-Hydroxybutyrate E. Succinate 14. In B-oxidation the sequence of intermediate are: alkane, alkene, alcohol, ketone. Where have we seen this sequence before? A) In the Kreb's cycle. D) In gluconeogenesis. B) In the urea cycle. E) In electron transport. C) In glycolysis.arrow_forwardIf you look at the minor metabolites, do they result from the same metabolic reactions/phases that glucuronide and sulfate are formed by? HO (2) Labetalol CH, Main metabolites: glucuronide and sulfate HO HN. NH, CH, Minor metabolite: 3-amino-1-phenyl butane CH, Minor metabolite 1 HN CH Minor metabolite 2arrow_forwardIn the body, during the citric acid cycle the following reaction occurs as the first part of an enzyme mediated process. но OH YYYYYY OH OH H₂O NaBH4 LiAlH4 H₂SO4 and heat K₂Cr2O7 HQ OH What reagent would be used to bring about this reaction in the laboratory? OH 3arrow_forward
- What is meant by peptization? Give a suitable example.arrow_forward3. Which of the following statements is FALSE? A. Enzymes speed up the attainment of a reaction equilibrium. B. Enzymes make reactions 10³ to 1020 times faster. C. Enzymes are proteins. D. Enzymes lower the amount of energy needed for a reaction. E. Enzymes are chemically unchanged during the actual catalytic process. 4. The polar head of cerebroside in the membrane can form a. Hydrogen bonds b. lon-dipole interactions c. Both A and B d. Neither A nor B with water molecule.arrow_forwardCarbohydrate Esterification What effects does the catalyst have on the product? What is the stoicheometry of the reaction?arrow_forward
- 5) A certain aerobic organism is able to metabolize the following glycolipid: "CH,OH H OH но OH A. Draw the 2 resulting structures that would occur upon initial hydrolysis of the 0-glycosidic bond. B. Calculate how much ATP is formed upon complete aerobic oxidation of one mole of the compound. Assume that no ATP is produced when one mole of the glycosidic bond in the above compound is hydrolyzed. Show calculation below.arrow_forwardWhich of the following has the highest iodine number? A. 1-oleyl-2-arachidonyl-3-palmitoyl glyceride B. 1-lauroyl-2,3-di-linolenyl glyceride C. 1-myritoleyl-2-stearoyl-3-linoleyl glyceride D. 1-palmitoleyl-2,3-arachidoyl glyceride E. 1,2,3-tri-oleyl glyceridearrow_forwardThe tartaness of some wines is high concentration of l malate.write a consequence of reactions showing how yeast cells synthesize l malate from glucose under anaerobic conditions in the presence of co2arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
