
Concept explainers
(a)
Interpretation: The given molecules need to be labeled as cis, trans or neither. The type of position of H atom circled in the given diagram needs to be described.
Concept Introduction: Chair conformation is the most stable conformation of cyclohexane. It is represented as follows:

Here, the substituted groups in the chair conformation are represented as follows:
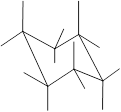
The groups showing in straight upward and downward directions are axial and the groups bend slightly right or left are equatorial.
(b)
Interpretation: The picture of the molecule needs to be drawn after the chair flip.
Concept Introduction:
The chair conformation of cyclohexane is represented as follows:

During the flipping, no bond is break. The numbering in the chair form is represented as follows:
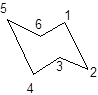
During ring flipping, mirror image of the chair conformation is formed.
It is represented as follows:
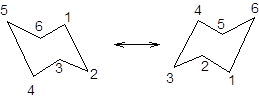
(c)
Interpretation: In each of the molecule of part (b), the conformation needs to be circled which is lower in potential energy.
Concept Introduction: The molecule with lowest potential energy is most stable. In the chair conformation of the cyclohexane, the stable conformation is when large groups are present in the equatorial positions. The equatorial positions in the chair conformation points away from the ring thus, there is more space.
(d)
Interpretation: The name of the two molecules drawn in part (b) needs to be determined
Concept Introduction: Chair conformation is the most stable conformation of cyclohexane. It is represented as follows:

Here, the substituted groups in the chair conformation are represented as follows:
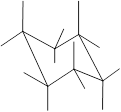
The groups showing in straight upward and downward directions are axial and the groups bend slightly right or left are equatorial.
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry: A Guided Inquiry
- Draw the “chair twist" of the following molecules. Label the more stable conformation. Br •CH3 OH Draw planar models of the above molecules. Draw both chair conformation of the following molecule, and identify the most stable form. Me Me ....Et Mearrow_forwardSight along the C2-Cl bond of 2-methylpropane (isobutane). a. Draw a Newman projection of the most stable conformation. b. Draw a Newman projection of the least stable conformation. c. Make a graph of energy versus angle of rotation around the C2-Cl bond. d. Assign relative values to the maxima and minima in your graph, given that an H↔H eclipsing interaction costs 0 kJ/mol and an H↔CH3 eclipsing interaction costs 6.0 kJ/mol.arrow_forwardC. Exercises 1. Which molecule representation best shows the bond interactions? a. Wedge and dash b. Sawhorse c. Newman d. Fischer Which molecule representation best combines the 3D molecule and the bond interactions? a. Wedge and dash b. Sawhorse c. Newman d. Fischer 3. Which molecule representation best showws the 3D model? a. Wedge and dash b. Sawhorse c. Newman d. Fischer 4. Staggered and eclipsed conformations are best shown using which projection? a. Wedge and dash b. Sawhorse c. Newman d. Fischer 5. Which is a more stable conformation? a. Eclipsed ethane b. Staggered ethanearrow_forward
- 7. Draw curved arrows indicating the movements of electrons between the following pair of resonance structures. Name the pattern of resonance shown. What is the hybridization of the carbon atoms? 8. Draw the remaining three resonance structures for the molecule in problem 7 above. 9. There are several possible forms of a trisubstituted cyclohexane with the formula C10H200. I have drawn four of them. From these, which one do you think is most commonly naturally occurring, and why? Which is least commonly occurring and why? HO HO" HO HOarrow_forwardF) Circle the letter corresponding to the relationship of each pair of structures: Identical (I), Conformers (C), Enantiomers (E) (enantiomers can have different conformations). FIRST... determine the absolute configuration of chiral centers. H. ІН' I H | H с H Me E Mearrow_forwardH3C H. NHCH3 NHCH3 H Harrow_forward
- Construct a model in which a tetrahedral carbon atom has four different colored model atoms attached to it- red, green, orange and white representing 4 different atoms attached to the central atom. a) Does the atom have a plane of symmetry? why or why not? b) Now replace the green atom in your model with a second orange atom. Now two of the groups attached to the carbon atom are identical. Does the model now have a plane of symmetry? Describe it. c)A carbon atom has four different groups attached to the stereogenic center. Draw structural formulas for the following compound and mark stereogenic centers with as asterisk: 1-bromobutane, 2-bromobutane, 1,2-dibromobutane, 1,4-dibromobutane, 2,3-dibromobutane.arrow_forward3. Draw the chair conformer of cyclohexane. Label the axial hydrogens (Ha) and the equatorial hydrogens (He).arrow_forwardDetermine whether each Newman projection is in the staggered or eclipsed conformation. Choose.. CH3 H. H. Choose... - CI CH3 H. Choose..- CH3 H. H. Choose.. CI CH3 ( BACK Question 5 of 7 archarrow_forward
- OH a. Draw the cis and trans isomer of the molecule above. b. Draw the chair form of the trans isomer of the molecule. C. Draw the flipped chair form of the trans isomer of the molecule. d. What is the more stable chair form?arrow_forwardHow are the molecules below related to each other? (Hint: convert each to a partially condensed structual formula). Н. H H CH3 H₂C CH3 CH3 and H H H H₂c-CH₂ CH3 H CH3 Select one: OA. They are constitutional isomers. OB. They are identical compounds. O C. They are geometrical isomers. O D. They are stereoisomers. O E. They are different compounds, not isomers..arrow_forward4. For the following structures, draw both possible chair conformations, using a ring flip to go from one to the other, and circle the one that is most stable/lowest energy. (Hint: Use Table 4.8 in Klein.) Show all groups, including hydrogen atoms, attached to the cyclohexane ring. А. Me Me Et В. Et t-Bu Etarrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
