
Organic Chemistry (6th Edition)
6th Edition
ISBN: 9781260119107
Author: Janice Gorzynski Smith
Publisher: McGraw Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8.5, Problem 12P
What
a.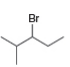 b.
b.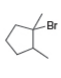 c.
c.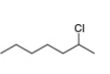 d.
d.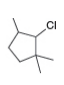
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Synthesize each compound from benzene and any other organic or inorganic reagents.
NH2
NO2
Br.
a.
е.
NH2
Br
CH3
Br
NH2
b.
d.
HOOC
NO2
(РАВA)
sunscreen component
What alkenes are formed from each alkyl halide by an E2 reaction? Label the major and minor products
Hint: Remember Zaitsev's rule!
a.
Br
b.
Br
C.
O#1 Draw all possible constitutional isomers formed by DEHYDROHALOGENATION of each alkyl halide.
Br
CI
Br
b.
а.
С.
d.
Chapter 8 Solutions
Organic Chemistry (6th Edition)
Ch. 8.1 - Problem 8.1 Label the and carbons in each alkyl...Ch. 8.2 - Problem 8.2 Classify each alkene in the following...Ch. 8.2 - Prob. 3PCh. 8.2 - Prob. 4PCh. 8.2 - Problem 8.5 Label each pair of alkenes as...Ch. 8.2 - Problem 8.6 Which alkene in each pair is more...Ch. 8.2 - Problem 8.7 Several factors can affect alkene...Ch. 8.4 - Prob. 8PCh. 8.4 - Prob. 9PCh. 8.4 - Prob. 10P
Ch. 8.4 - Prob. 11PCh. 8.5 - Problem 8.12 What alkenes are formed from each...Ch. 8.6 - Prob. 13PCh. 8.6 - Problem 8.14 What alkenes are formed from each...Ch. 8.6 - Problem 8.15 How does each of the following...Ch. 8 - 8.24 Rank the alkenes shown in the ball-and-stick...Ch. 8 - Prob. 25PCh. 8 - 8.26 What is the major E2 elimination product...Ch. 8 - Prob. 27PCh. 8 - Prob. 28PCh. 8 - Prob. 29PCh. 8 - 8.30 Label each pair of alkenes as constitutional...Ch. 8 - Prob. 31PCh. 8 - Prob. 32PCh. 8 - Prob. 33PCh. 8 - For each of the following alkenes, draw the...Ch. 8 - Prob. 35PCh. 8 - Prob. 36PCh. 8 - Prob. 37PCh. 8 - What alkene is the major product formed from each...Ch. 8 - Prob. 39PCh. 8 - Prob. 41PCh. 8 - Draw the products formed when each dihalide is...Ch. 8 - Draw all of the substitution and elimination...Ch. 8 - Prob. 56PCh. 8 - 8.59 Draw a stepwise, detailed mechanism for each...Ch. 8 - Draw the major product formed when...Ch. 8 - Draw a stepwise, detailed mechanism for the...Ch. 8 - Explain why the reaction of with gives ...Ch. 8 - Draw a stepwise detailed mechanism that...Ch. 8 - Prob. 63PCh. 8 - 8.65 Explain the selectivity observed in the...Ch. 8 - Prob. 65PCh. 8 - Prob. 66PCh. 8 - 8.68 (a) Draw all products formed by treatment of...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Q1. What is the empirical formula of a compound with the molecular formula
Chemistry: A Molecular Approach
Fully developed conditions are known to exist for water flowing through a 25-nim-diameer tube at 0.01 kg/s and ...
Fundamentals of Heat and Mass Transfer
Practice Problem 1.22 Which of the following alkenes can exist as cis-trans isomers? Write their structures. Bu...
Organic Chemistry
Real walls are never totally adiabatic. Use your experience to order the following walls in increasing order wi...
Thermodynamics, Statistical Thermodynamics, & Kinetics
2. Why shouldn’t you work in a laboratory by yourself?
The Organic Chem Lab Survival Manual: A Student's Guide to Techniques
45. Calculate the mass of nitrogen dissolved at room temperature in an 80.0-L home aquarium. Assume a total pre...
Chemistry: Structure and Properties
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- 1. Draw the products of each nucleophilic substitution reaction a. b. D b OH C. d. e. f. Br 1 NaCN + NaOCH3 H₂Oarrow_forwardDevise a synthesis of each compound using CH3CH₂CH=CH₂ as the starting material. You may use any other organic compounds or inorganic reagents. a. b. d. e. Br Br Br OH OH (+ enantiomer)arrow_forwardSynthesize each compound from toluene and any other organic or inorganic reagents. a. C6HSCH2BR b. C6HSCH2OC(CH3)3 -CHO с. O,N НООС -NO2 d. H,N.arrow_forward
- Which reaction will produce this alkene as the major product? A. A B. B C. Both D. Neither (a) (b) Br NaOCH3 Δ H₂C P(Ph)3arrow_forwardSynthesize each compound from cyclohexanone and organic halides having s4 C's. You may use any other inorganic reagents. OCH3 a. C. OH b. d.arrow_forwardQuestion 8: Draw the products formed when each diene is treated with 03 followed by CH3SCH3? a. b. C. d. Question 9: Draw the products formed when each alkyne is treated with 03 followed by H₂O.arrow_forward
- These reagents can produce ketones with alkynes A. BH3, THF, H2O2 B. KMnO4 C. O3 D. H2SO4, H2O, HgSO4arrow_forwardPredict the oxidation product of treating the given alkene with a peroxyacid reagent. Omit byproducts. Draw the oxidation product. H. Harrow_forward6. Give the structures of E2 reaction products of the following alkyl halides with a strong base. a a. 11 CI b. III Clarrow_forward
- Synthesis 10.63 Devise a synthesis of each product from the given starting material. More than one step is required. a. b. d. e. Br Br OH OCH3 ta CI OHarrow_forward5. What reagents are needed to convert toluene (C,H,CH,) to each compound? a. C.H.COOH b. C.H₂CH₂Br c. p-bromotoluene d. o-nitrotoluene e. p-ethyltoluene f.arrow_forwardWhat alkenes are formed from each alkyl halide by an E2 reaction? Use the Zaitsev rule to predict the major product.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY