
Concept explainers
(a)
Interpretation:
The formal charge on each atom and the net charge in the given species are to be stated.
Concept introduction:
Chemical compounds contain two types of bonds. These are known as ionic and covalent bonds. In ionic bonds, the ions are held by the electrostatic interaction between them. In covalent bonds, the atoms are held together by the sharing of electrons. The formal charge is the charge on the constituent atoms in a molecule. It is calculated by using valence electrons of the atom.
Answer to Problem 1.25AP
The formal charges on oxygen atom and chlorine atom are
Explanation of Solution
The given structure is shown in figure 1.
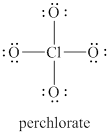
Figure 1
The formal charge on atom is calculated by the formula given below as,
The valence electrons of oxygen atoms in given species are
Thus, the formal charge on each oxygen atom is
The valence electrons of chlorine atom in given species are
Thus, the formal charge on chlorine atom is
The total charge on the given species is calculated as follows.
Hence, total charge on the species is
The formal charges on oxygen atom and chlorine atom are
(b)
Interpretation:
The formal charge on each atom and the net charge in the given species are to be stated.
Concept introduction:
Chemical compounds contain two types of bonds. These are known as ionic and covalent bonds. In ionic bonds, the ions are held by the electrostatic interaction between them. In covalent bonds, the atoms are held together by the sharing of electrons. The formal charge is the charge on the constituent atoms in a molecule. It is calculated by using valence electrons of the atom.
Answer to Problem 1.25AP
The formal charges on oxygen atom, nitrogen atom and carbon atom are
Explanation of Solution
The given structure is shown in figure 2.
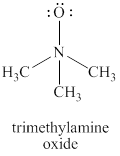
Figure 2
The formal charge on atom is calculated by the formula given below as,
The valence electrons of oxygen atoms in given species are
Thus, the formal charge on each oxygen atom is
The valence electrons of nitrogen atom in given species are
Thus, the formal charge on nitrogen atom is
The valence electrons of carbon atom in given species are
Thus, the formal charge on carbon atom is
The total charge on the given species is calculated as follows.
Hence, total charge on the species is
The formal charges on oxygen atom, nitrogen atom and carbon atom are
(c)
Interpretation:
The formal charge on each atom and the net charge in the given species are to be stated.
Concept introduction:
Chemical compounds contain two types of bonds. These are known as ionic and covalent bonds. In ionic bonds, the ions are held by the electrostatic interaction between them. In covalent bonds, the atoms are held together by the sharing of electrons. The formal charge is the charge on the constituent atoms in a molecule. It is calculated by using valence electrons of the atom.
Answer to Problem 1.25AP
The formal charges on left oxygen atom, right oxygen atom and central oxygen atom are
Explanation of Solution
The given structure is shown in figure 3.
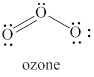
Figure 3
The formal charge on atom is calculated by the formula given below as,
The valence electrons of left oxygen atom in given species are
Thus, the formal charge on each oxygen atom is
The valence electrons of right oxygen atom in given species are
Thus, the formal charge on each oxygen atom is
The valence electrons of central oxygen atom in given species are
Thus, the formal charge on each oxygen atom is
The total charge on the given species is calculated as follows.
Hence, total charge on the species is
The formal charges on left oxygen atom, right oxygen atom and central oxygen atom are
(d)
Interpretation:
The formal charge on each atom and the net charge in the given species are to be stated.
Concept introduction:
Chemical compounds contain two types of bonds. These are known as ionic and covalent bonds. In ionic bonds, the ions are held by the electrostatic interaction between them. In covalent bonds, the atoms are held together by the sharing of electrons. The formal charge is the charge on the constituent atoms in a molecule. It is calculated by using valence electrons of the atom.
Answer to Problem 1.25AP
The formal charge on carbon atom is
Explanation of Solution
The given structure is shown in figure 4.
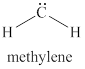
Figure 4
The formal charge on atom is calculated by the formula given below as,
The valence electrons of carbon atom in given species are
Thus, the formal charge on carbon atom is
Hence, total charge on the species is also
The formal charge on carbon atom is
(e)
Interpretation:
The formal charge on each atom and the net charge in the given species are to be stated.
Concept introduction:
Chemical compounds contain two types of bonds. These are known as ionic and covalent bonds. In ionic bonds, the ions are held by the electrostatic interaction between them. In covalent bonds, the atoms are held together by the sharing of electrons. The formal charge is the charge on the constituent atoms in a molecule. It is calculated by using valence electrons of the atom.
Answer to Problem 1.25AP
The formal charge on carbon atom and carbon radical is
Explanation of Solution
The given structure is shown in figure 5.
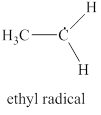
Figure 5
The formal charge on atom is calculated by the formula given below as,
The valence electrons of carbon atom in given species are
Thus, the formal charge on carbon atom is
The valence electrons of carbon radical in given species are
Thus, the formal charge on carbon radical is
Hence, total charge on the species is also
The formal charge on carbon atom and carbon radical is
(f)
Interpretation:
The formal charge on each atom and the net charge in the given species are to be stated.
Concept introduction:
Chemical compounds contain two types of bonds. These are known as ionic and covalent bonds. In ionic bonds, the ions are held by the electrostatic interaction between them. In covalent bonds, the atoms are held together by the sharing of electrons. The formal charge is the charge on the constituent atoms in a molecule. It is calculated by using valence electrons of the atom.
Answer to Problem 1.25AP
The formal charge on oxygen atom and chlorine atom is
Explanation of Solution
The given structure is shown in figure 6.
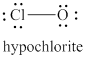
Figure 6
The formal charge on atom is calculated by the formula given below as,
The valence electrons of oxygen atoms in given species are
Thus, the formal charge on each oxygen atom is
The valence electrons of chlorine atom in given species are
Thus, the formal charge on chlorine atom is
The total charge on the given species is calculated as follows.
Hence, total charge on the species is
The formal charge on oxygen atom and chlorine atom is
Want to see more full solutions like this?
Chapter 1 Solutions
Organic Chemistry
- Compute the formal charge (FC) on each atom in the following structures. (b) The hydronium ion, H3O+arrow_forward16. Which of the following structures is the CORRECT resonance structure of the following. molecule: (A) (B) (C) (D) CH3- CH₂ CH3 -H CH₂CH3 CH₂CH-CH₂ CH3 CH3arrow_forwardConsider Lewis formulas A, B, and C:(a) Are A, B, and C constitutional isomers, or are they resonance contributors? (b) Which have a negatively charged carbon? (c) Which have a positively charged carbon? (d) Which have a positively charged nitrogen? (e) Which have a negatively charged nitrogen? (f) What is the net charge on each? (g) Which is a more stable structure, A or B? Why? (h) Which is a more stable structure, B or C? Why? (i) What is the CNN geometry in each according to VSEPR?arrow_forward
- 6) Which of the following pairs of compounds are resonance structures? CH3 CH3 CH2 CH3 CH3 CH3 and and and (1) (II) (III) CH3 CH3 CH3 and and (IV) (V) (a) I (b) I| (c) III (d) IV (e) Varrow_forwardDraw the shapes of the following molecules and ions in 3-dimension. Show clearly any lone pairs of electrons on the central atom, state the number of bond pairs and lone pairs of electrons on the central atom and name the shape of the molecule or ion. (a) AIH4-, aluminium hydride ion (b) CH3-, methyl carbanion (c) POCl3, phosphorus oxychloride Provide everything stated in the instructions for each compound.arrow_forwardBelow are a series of potential resonance structures, along with the curved-arrows that translate between them. Which of the following is NOT a valid pair of resonance structures? 3 - 2 (A) (B) (C) (D) • Rectangular Sniparrow_forward
- For each of the following structures,1. Draw a Lewis structure; fill in any nonbonding electrons.2. Calculate the formal charge on each atom other than hydrogen.(a) CH3NO(nitromethane)(b) (CH3)3NO(trimethylamine oxide)(c) [N3]-(azide ion)(d) [(CH3)3O]+ (e) CH3NC (f) (CH3)4NBrarrow_forwardPlease provide the formal charge of the above question. c)arrow_forwardConsider compounds A–D, which contain both a heteroatom and a double bond. (a) For which compounds are no additional Lewis structures possible? (b) When two or more Lewis structures can be drawn, draw all additional resonance structures.arrow_forward
- Draw Lewis structures for each of the following compounds. In each case, specify the number of valence electrons surrounding the central atom. (Assign lone pairs and radical electrons where appropriate.) (Assume the central atom does not contain an expanded octet.) (a) bromine dioxide (BrO2) (b) beryllium bromide (BeBr2) (c) phosphorus pentafluoride (PF5)arrow_forward19.) The thiocyanate ion (SCN-) has thee resonance structures. Each follows the octet rule (#1) has a sulfur to carbon single bond and a carbon to nitrogen triple bond. (#2) has a sulfur to carbon triple bond and a carbon to nitrogen single bond. (#3) has a sulfur to carbon double bond and a carbon to nitrogen double bond. Which structure is the "best" one using formal charge arguments? Group of answer choices 2 all three are equal 1 3 1 and 2 are equalarrow_forwardDraw a structural formula for a hydrocarbon with the given molecular formula that undergoes hydroboration-oxidation to give the indicated product. (a) (b) • All hydrogen atoms are implied. Apply formal charges where appropriate. • Omit lone pairs and radical electrons from your answer. ● C₂H10 C₂H12 1. (sia) BH 2. H₂O₂, NaOH Il 1. BH₂ 2. H₂O2₂, NaOH H Sn [F ? ChemDoodle OHarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





