
Essential Organic Chemistry (3rd Edition)
3rd Edition
ISBN: 9780321937711
Author: Paula Yurkanis Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 11.12, Problem 22P
Interpretation Introduction
Interpretation:
The mechanism has to be written for the reaction of an amide with an alcohol in presence of an acid catalyst to give ester as a product.
Concept introduction:
The general reaction of an amide with an alcohol does not give a product. As amides are least reactive
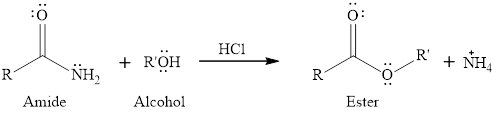
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Benzoic acid reacts with ethylamine to form what kind of amide
What are the products of hydrolysis of an ester in the presence of a base?
An amidification reaction is the reaction of a carboxylic acid with an amine (or ammonia) to produce an amide. Draw the structure of the carboxylic acid from which the following amide could be formed.
Chapter 11 Solutions
Essential Organic Chemistry (3rd Edition)
Ch. 11.1 - The aromas of many flowers and fruits are due to...Ch. 11.1 - Name the following compounds:Ch. 11.1 - Prob. 3PCh. 11.2 - Prob. 4PCh. 11.2 - Prob. 5PCh. 11.4 - a. What is the product of the reaction of acetyl...Ch. 11.4 - Prob. 7PCh. 11.5 - Using the pKa values listed in Table 11.1, predict...Ch. 11.6 - Starting with acetyl chloride, what neutral...Ch. 11.6 - Prob. 10P
Ch. 11.7 - Prob. 11PCh. 11.8 - Prob. 13PCh. 11.8 - Using the mechanism for the acidcatalyzed...Ch. 11.8 - Prob. 15PCh. 11.8 - Prob. 16PCh. 11.8 - Prob. 17PCh. 11.9 - Prob. 18PCh. 11.10 - Show how each of the following esters could be...Ch. 11.11 - Which of the following reactions would lead to the...Ch. 11.12 - Prob. 22PCh. 11.12 - Prob. 23PCh. 11.13 - Prob. 24PCh. 11.13 - Prob. 25PCh. 11.14 - Prob. 26PCh. 11.14 - Prob. 27PCh. 11.14 - Prob. 28PCh. 11.15 - Prob. 29PCh. 11.15 - How would you synthesize the following compounds...Ch. 11 - Write a structure for each of the following a. N,N...Ch. 11 - Prob. 32PCh. 11 - Which ester is more reactive, methyl acetate or...Ch. 11 - What products would be formed from the reaction of...Ch. 11 - What products would be obtained from the following...Ch. 11 - Prob. 36PCh. 11 - a. Which compound would you expect to have a...Ch. 11 - a. List the following esters in order of...Ch. 11 - D. N. Kursanov, a Russian chemist, proved that the...Ch. 11 - Prob. 40PCh. 11 - Using an alcohol for one method and an alkyl...Ch. 11 - Prob. 42PCh. 11 - Prob. 44PCh. 11 - Prob. 45PCh. 11 - Prob. 46PCh. 11 - Prob. 47PCh. 11 - Prob. 48PCh. 11 - Prob. 49PCh. 11 - Show how the following compounds could be prepared...Ch. 11 - Prob. 51PCh. 11 - Prob. 52PCh. 11 - Prob. 53P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- explain why an N,N-disubstituted amide is less acidic than an ester.arrow_forwarddraw the condensed structural formula for the product from the hydrolysis of each of the following amides with HClarrow_forwardDraw the organic product formed when these compounds undergo a substitution reaction butanoic acid and 2-propanol acetic acid and methylamine formic acid and 2-methyl-1-propanolarrow_forward
- Acid Anhydride Amine Thiol Peroxide Nitrile Arenearrow_forwardHow a Carboxylic Acid and an Amine Undergo an Acid–Base Reaction?arrow_forwardMany substitution reactions are initiated by electrostatic attraction between reactants. Show where this attraction arises in the formation of an amide from an amine and an ester.arrow_forward
- Describe the product formed as a result of the reaction between benzoic acid and ethyl alcohol in an acidic environment by writing the mechanism of the reaction.arrow_forwardWhat is the role of pyridine in the acylation reaction of amines?arrow_forwardDraw the products of acid–base reactions of aminesarrow_forward
- Write a general reaction showing the preparation of an ester.arrow_forwardName the sodium salt of the carboxylic acid that is formed by the saponification (basic hydrolysis) of the following ester with sodium hydroxide:arrow_forwardName (by the common system of nomenclature) the amine (or ammonia) that is formed by the basic hydrolysis of the following acid amide:arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,