
Organic Chemistry
9th Edition
ISBN: 9781305080485
Author: John E. McMurry
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 12.2, Problem 2P
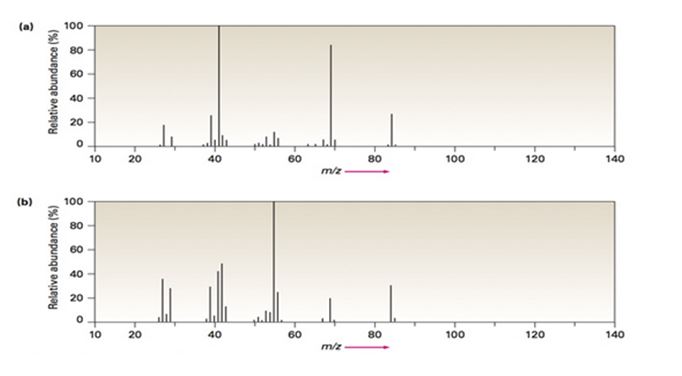
Two mass spectra are shown in FIGURE 12-8. One spectrum is that of 2-methyl-2-pentene; the other is of 2-hexene. Which is which? Explain.
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
Absorption and emission of electronic energy:
a) You find two bottles in the lab that have lost their labels. You know that the two
compounds are 2,3-dimethyl-1,3-butadiene and 2,5-dimethyl-1,3,5-hexatriene. You
find that UV absorbance maxima are at 226 nm and 252 nm. Which spectrum belongs
to which compound? How do you know this?
H.
H
Segment of
a sheet of
graphite
CH3
CH3
H
b) The (n to *) transition of N=0 has a maximum at 660 nm, while the ( to *) transition in C=C is at 180 nm.
Why would the transition for the N=O molecule be at a much longer wavelength? You may wish to use simple
HOMO and LUMO energy diagrams for clarity.
CH₂
c) Diamond and graphite are allotropic forms of carbon. Consider the molecular structures shown and the types
of bonds. Use them to explain why graphite (LEFT) is black while diamonds (RIGHT) are colourless.
Segment of
a diamond
a. Draw and name all of the isomeric products obtainedfrom the monobromination of propane with Br2/light. If halogenation were a completely randomreaction and had an equal probability of occurring atany of the C—H bonds in a molecule, what percentage of each of these monobromo products would beexpected?b. Answer part (a) using 2-methylpropane as the starting material.
Provide the structures (line, expanded structural or condensed structural formulas) for boxes
1-17 (there is no 13).
1
2
CH;OH
CH;OH
H*
H*
hemiketal
ketal
3
OH
H.
H*
H*
hemiacetal
acetal
6.
OH
OH
H*
H*
hemiketal
ketal
CH;OH
(excess)
H2SO4
8
CH3OH
(excess)
H2SO4
Chapter 12 Solutions
Organic Chemistry
Ch. 12.2 - Prob. 1PCh. 12.2 - Two mass spectra are shown in FIGURE 12-8. One...Ch. 12.3 - What are the masses of the charged fragments...Ch. 12.3 - Prob. 4PCh. 12.5 - Prob. 5PCh. 12.5 - Prob. 6PCh. 12.7 - What functional groups might the following...Ch. 12.7 - How might you use IR spectroscopy to distinguish...Ch. 12.8 - Prob. 9PCh. 12.8 - Where might the following compounds have IR...
Ch. 12.8 - Where might the following compound have IR...Ch. 12.SE - Prob. 12VCCh. 12.SE - Show the structures of the fragments you would...Ch. 12.SE - Propose structures for compounds that fit the...Ch. 12.SE - Write molecular formulas for compounds that show...Ch. 12.SE - Camphor, a saturated monoketone from the Asian...Ch. 12.SE - The nitrogen rule of mass spectrometry says that a...Ch. 12.SE - In light of the nitrogen rule mentioned in Problem...Ch. 12.SE - Nicotine is a diamino compound isolated from dried...Ch. 12.SE - The hormone cortisone contains C, H, and O, and...Ch. 12.SE - Halogenated compounds are particularly easy to...Ch. 12.SE - Prob. 22APCh. 12.SE - Propose structures for compounds that fit the...Ch. 12.SE - 2-Methylpentane (C6H14) has the mass spectrum...Ch. 12.SE - Assume that you are in a laboratory carrying out...Ch. 12.SE - What fragments might you expect in the mass...Ch. 12.SE - How might you use IR spectroscopy to distinguish...Ch. 12.SE - Would you expect two enantiomers such as...Ch. 12.SE - Would you expect two diastereomers such as meso-2,...Ch. 12.SE - Propose structures for compounds that meet the...Ch. 12.SE - How could you use infrared spectroscopy to...Ch. 12.SE - Prob. 32APCh. 12.SE - At what approximate positions might the following...Ch. 12.SE - How would you use infrared spectroscopy to...Ch. 12.SE - At what approximate positions might the following...Ch. 12.SE - Assume that you are carrying out the dehydration...Ch. 12.SE - Assume that you are carrying out the base-induced...Ch. 12.SE - Prob. 38APCh. 12.SE - Carvone is an unsaturated ketone responsible for...Ch. 12.SE - Prob. 40APCh. 12.SE - The mass spectrum (a) and the infrared spectrum...Ch. 12.SE - The mass spectrum (a) and the infrared spectrum...Ch. 12.SE - Propose structures for compounds that meet the...Ch. 12.SE - 4-Methyl-2-pentanone and 3-methylpentanal are...Ch. 12.SE - Grignard reagents undergo a general and very...Ch. 12.SE - Ketones undergo a reduction when treated with...Ch. 12.SE - Nitriles, R–=C≡N, undergo a hydrolysis...Ch. 12.SE - The infrared spectrum of the compound with the...Ch. 12.SE - The infrared spectrum of the compound with the...Ch. 12.SE - Prob. 50AP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- In light of the nitrogen rule mentioned in Problem 12-17, what is the molecular formula of pyridine, M+=79?arrow_forward3. Four part question. (i) names are these structures (ii) how do they contribute to aroma (iii) which hops varieties have these compounds and (iv) what are their aroma thresholds? CH3 H2C H ", CH3 CH3 CH2 CH2 H3C CH3arrow_forward10. Fill the blanks. Name Methoxyethane Ethyl acetate Dime 2-Bromopropane 1,4-Dibromobutane Acetone 3-Methylpentane 1,2- Dichloeopropane Ethanol 2-Chloro-2- methylbutane Structure År Xu Br Possible chemical shift for each unique peak Integral Ratio between (among) peaksarrow_forward
- What is the complete IUPAC name of the following structure? lC2H5 H3C CH3 A. (1R)-1-ethyl-3,3-dimethylcyclopentane B. (1S)-1-ethyl-3,3-dimethylcylcopentane C. (1R,3S)-1-ethyl-3,3-dimethylcyclopentane D. (1S,3R)-1-ethyl-3,3-dimethylcylopentanearrow_forwardI have the following data from an NMR scan. Five peaks; a doublet at about 0.8ppm (6H) a small single septet at about 1.5 ppm (1H), a triplet at 1.6 ppm (2H(, a singlet at about 2.1 ppm (3H), a triplet at about 4.4 ppm (2H). The molecular formula is C7H14O2. I calculated a degree of unsaturation of 1. I have tentatively identified the compound as methyl-4-methylbutanoate. I would appreciate any comments you care to make. Thank you.arrow_forwardFor the substituted cyclohexane compound given below, highlight the groups – by clicking on atoms – that are trans to the bromo substituent. CH3 Br H. но H.arrow_forward
- With the molecular formula C4H8O , I ask you to draw 3 molecules: an aldehyde, a ketone and an enol.For each molecule, you must: a) Complete detailed Lewis structure;b) Make the 3D structure respecting the connection angles;c) Determine which intermolecular interactions are present if you glue 2 twin molecules together (2 aldehydes together, 2 ketones together, 2 enols together, therefore 3 situations to analyze).arrow_forwardC|Gb|c|c|OU| 3 > |||| F3 > > $ BW BAX G d H 2 bA|QJ|G1|CS|UONEE Question 8 of 31 Which molecular formula corresponds to an alkene? A) CH₁0 6 10 B) C₂H₁4 14 C) CH₂ D) C₁0H₂2 10 22 E) C₂H. 2 6 4 V Q F4 Aa ✓ % 5 T 0 0 B Ų F5 A 6 MacBook Air c F6 & 7 2 F7 8 DII F8 ( 9 F9 C O v F10 Done P BG C F11arrow_forwardPlease do the following question. Step by step A. 1-butyne + HgSO4, H2SO4 and H2O B. 2-methyl-1,3-butadiene + HC ≡ C – C ≡ Narrow_forward
- For the substituted cyclohexane compound given below, highlight the groups – by clicking on atoms – that are cis to the hydroxyl (OH) substituent. CH3 Br F. ноarrow_forward3. Use curve-arrow notation to show electron flow in each of the following reactions and draw the structure of the product formed in each instance. a) HO 10: h) 10: cate :0: :0: :OH علق OH :0: HIC CH :OCH, O.. OH ww OH BOH NH, + Nall + HCO, + :NH₂arrow_forward2 F2 W Provide the correct IUPAC name for the compound shown here. # 3 F3 e Oll F4 $ 4 1,3- ·0. F5 do in Question 23 of 49 iso t para- ortho- meta- 1,5-1-3- M F6 Br A 6 31 tert- di cvclo C DELL y CI F7 & 7 5- 1,3,5- 3 F8 * 8 F9 9 D F10 O 0 F11 Р 8 Mar 2 F12 + { [arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY