
Concept explainers
Interpretation:
Four isomers consistent with the formula
Concept introduction:
The IHD accounts for the number of rings or multiple bonds the structure could possess.
Number of conjugated C=C double bond are predicted using following table.
| Conjugated |
UV |
Change in |
| - | ||
Answer to Problem 15.32P
Four isomers consistent with the formula
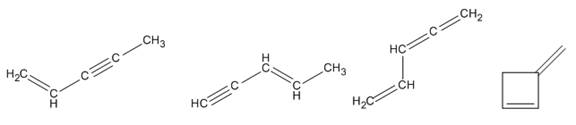
Explanation of Solution
The given molecular formula is
From the table
| Conjugated |
UV |
Change in |
| - | ||
The two conjugated
Therefore four isomers consistent with the formula
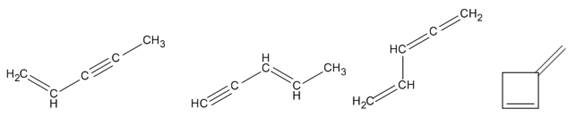
Isomers consistent with the formula
Want to see more full solutions like this?
Chapter 15 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- The structure of the molecule cyclohexene is Does the absorption of ultraviolet light by cyclohexene occur at longer or at shorter wavelengths than the absorption by benzene? Explain.arrow_forwardView and analyze the given spectroscopic data of an unknown compound with molecular formula C8H10O. Propose a structure based on your analysis. Support your answer by interpreting the given spectroscopic data.arrow_forwardChoose the correct answer. Choose. Choose. An alkyne with molecular formula C5H10 A ketone with molecular formula C4H80 An aldehyde with molecular formula C2H4O A saturated hydrocarbon with molecular formula C6H14 An alkene with molecular formula C5H10 An alkene with molecular formula C5H8 A ketone with molecular formula C3H8O An aldehyde with molecular formula CH40 Choose.arrow_forward
- Calculating the Number of Degrees of Unsaturation in Compounds with O, X, or N Calculate the number of degrees of unsaturation for each molecular formula: (a) C5H8O; (b) C6H11Cl; (c) C8H9N. Propose one possible structure for each compound.arrow_forwardChoose an answer An alcohol with molecular formula C4H8O A Ketone with molecular formula C3h6O An alkane with molecular formula C6H14 An akene with molecular formula C5H10 An unsatrated hydrocarbon with molecular formula C6h12 An aldehyde with molecular formula C2H6O A Ketone with molecular formula C2H6Oarrow_forward000 000 Critical absorbance frequency(ies), cm1 Functional group Identity:arrow_forward
- Here is the chemical structure of 2-bromobutane: Н Η Н Н HHHH C C- C C-H H :Br Η Н .. Decide whether each molecule in the table below is another molecule of 2-bromobutane, a molecule of an isomer of 2-bromobutane, or a molecule of an entirely different compound. molecule CH₂ CH₂-CH₂-CH-Br CH₂ CH₂ -CH₂ Br HI H H H Η H_ H-C- C-C- H-C-H H H relationship to 2-bromobutane (Choose one) (Choose one) Br: a molecule of an isomer of 2-bromobutane ▼arrow_forwardFor which compound containing a heteroatom (an atom other than carbon or hydrogen) does the molecular ion have an even-numbered mass? For which does it have an odd-numbered mass? Q.) A bromoalkane with the molecular formula CnH2n11Brarrow_forwardDraw the structure for a compound that meets this description (can be any structure that meets this description): C3H6O, with no IR absorption above 3000 cm^-1.arrow_forward
- Draw and name the six isomeric cyclopentane of molecular formula C7H14. These will include four constitutional isomers, of which two show geometric (cis-trans) stereoisomerism.arrow_forwardIn addition to more highly fluorinated products, fluorination of 2-methylbutane yields a mixture of compounds with the formula C5H10F2. Draw the structures of all the isomers with the formula C5H10F2 that would be produced and label with a star all the chiral centers present in their structures.arrow_forwardMethionine is an amino acid used in the biosynthesis of proteins. The structural diagram for methionine is: H H H H H H H °N H H Using VSEPR theory, consider the stereochemical diagram that would form. Identify t geometric shape at the six identified locations on the above molecule of methionine. Hint: Treat each location as a separate central atom. Remember to add in lone pairs Review this example from your course. (Unit A Section 3 Lesson 8.2 - Digging Deeper) Geometric shape around atom 1 is tetrahedral Geometric shape around atom 2 is tetrahedral Geometric shape around atom 3 is tetrahedral Geometric shape around atom 4 is trigonal planar Geometric shape around atom 5 is tetrahedral Geometric shape around atom 6 is trigonal pyramidal +arrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


