
a)

Interpretation:
Starting from benzene or toluene and assuming that the ortho and para isomers can be separated, how 2-bromo-4-nitrotoluene can be synthesized is to be stated.
Concept introduction:
In
Electrophilic substitution of di and trisubstituted benzenes follows three simple rules. (i) If the directing influence of both the substituents reinforce each other, a single product results. (ii) If the directing influences of both the substituent groups oppose each other, the most powerful activating group among them has the dominant influence but usually a mixture of products results. (iii) In meta disubstituted compounds, further substitution in between the groups occurs only rarely, due to steric reasons.
To state:
Starting from benzene or toluene and assuming that the ortho and para isomers can be separated, how 2-bromo-4-nitrotoluene can be synthesized.
b)

Interpretation:
Starting from benzene or toluene and assuming that the ortho and para isomers can be separated, how 1,3,5-trinitrobenzene can be synthesized is to be stated.
Concept introduction:
In aromatic electrophilic substitution reactions the nitro group is a meta directing group. Nitration of benzene gives nitrobenzene. Further nitration of nitrobenzene will introduce nitro groups into the two meta positions to yield the trinitro compound.
To state:
Starting from benzene or toluene and assuming that the ortho and para isomers can be separated, how 1,3,5-trinitrobenzene can be synthesized.
c)
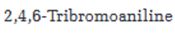
Interpretation:
Starting from benzene or toluene and assuming that the ortho and para isomers can be separated, how 2,4,6-tribromoaniline can be synthesized is to be stated.
Concept introduction:
In aromatic electrophilic substitution reactions, the amino group is an ortho and para directing group. Nitration of benzene gives nitrobenzene. Nitrobenzene can be reduced to aminobenzene (aniline). Aniline upon bromoination will yield 2,4,6-tribromoaniline.
To state:
Starting from benzene or toluene and assuming that the ortho and para isomers can be separated, how 2,4,6-tribromoaniline can be synthesized.
d)
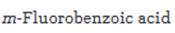
Interpretation:
Starting from benzene or toluene and assuming that the ortho and para isomers can be separated, how m-fluorobenzoic acid can be synthesized is to be stated.
Concept introduction:
In aromatic electrophilic substitution reactions the
To state:
Starting from benzene or toluene and assuming that the ortho and para isomers can be separated, how m-fluorobenzoic acid can be synthesized.
Trending nowThis is a popular solution!

Chapter 16 Solutions
Organic Chemistry
- Draw structural formulas for these compounds. (a) 1-Bromo-2-chloro-4-ethylbenzene (b) 4-Bromo-1,2-dimethylbenzene (c) 2,4,6-Trinitrotoluene (d) 4-Phenyl-1-pentene (e) p-Cresol (f) 2,4-Dichlorophenolarrow_forwardBiphenyl has the following structure.(a) Is biphenyl a (fused) polynuclear aromatic hydrocarbon?(b) How many pi electrons are there in the two aromatic rings of biphenyl? How does this number compare with that for naphthalene?arrow_forwardWrite structural formulas for the following compounds (includes both old- and new-style names).(a) 2-octyne (b) ethylisopentylacetylene (c) ethynylbenzene(d) cyclohexylacetylene (e) 5-methyl-3-octyne (f) trans-3,5-dibromocyclodecynearrow_forward
- Draw the structure of the following compounds which parent names have been traced to a common name; (a)5-methyl-4-nitroimidazole (b)2-chloro-4-methoxythiazole.arrow_forwardDraw the structures of the following compounds:(a) Ethanoic acid(b) Bromopentane(c) Butanonearrow_forwardGive reasons for the following :(i) Phenol is more acidic than methanol.(ii) The C—O—H bond angle in alcohols is slightly less than the tetrahedral angle (190°28′).(iii) (CH3)3C—O—CH3 on reaction with HI gives (CH3)3C—I and CH3—OH as the main products and not (CH3)3C—OH and CH3—I.arrow_forward
- Draw structural formulas for these ketones. (a) Ethyl isopropyl ketone (b) 2-Chlorocyclohexanone (c) 2,4-Dimethyl-3-pentanone (d) Diisopropyl ketone (e) Acetone (f) 2,5-Dimethylcyclohexanonearrow_forward(a) How will you convert:(i) Benzene to acetophenone (ii) Propanone to 2-Methylpropan-2-ol(b) Give reasons :(i) Electrophilic substitution in benzoic acid takes place at meta position.(ii) Carboxylic acids are higher boiling liquids than aldehydes, ketones and alcohols of comparable molecular masses.(iii) Propanal is more reactive than propanone in nucleophilic addition reactions.arrow_forwardShow the chemical reaction on how to convert cyclopentene into these compounds. (a) 1,2-dimethylcyclopropane (b) Cyclopentanol (c) Iodocyclopentane (d) Cyclopentane.arrow_forward
- (a) Draw the three isomers of benzenedicarboxylic acid.(b) The isomers have melting points of 210 °C, 343 °C, and 427 °C. Nitration of the isomers at all possible positions was once used to determine their structures. The isomer that melts at 210 °C gives two mononitro isomers. The isomer that melts at 343 °C gives three mononitro isomers. The isomer that melts at 427 °C gives only one mononitro isomer. Show which isomer has which melting point.arrow_forwardHow could you convert butanenitrile into the following compounds? Write each step showing the reagents needed. (a) 1-Butanol (b) Butylaminearrow_forward16-57 Starting with either benzene or toluene, how would you synthesize the following substances? Assume that ortho and para isomers can be separated. (a) 2-Bromo-4-nitrotoluene (c) 2,4,6-Tribromoaniline (b) 1,3,5-Trinitrobenzene (d) m-Fluorobenzoic acidarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





