
Concept explainers
(a)
Interpretation:
The structure of
Concept introduction:
Answer to Problem 21.1P
The structure of
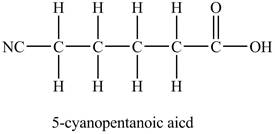
Explanation of Solution
In
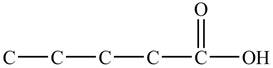
Figure 1
At the fifth carbon, there is a cyano group
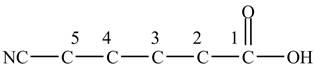
Figure 2
The above skeleton is completed by adding hydrogens as per the tetravalency of carbon as shown below.
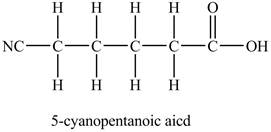
Figure 3
The structure of
(b)
Interpretation:
The structure of isopropyl valerate is to be stated.
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
Answer to Problem 21.1P
The structure of isopropyl valerate is shown below.
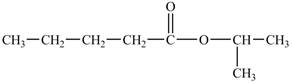
Explanation of Solution
The structure of isopropyl valerate consists of eight carbons, sixteen hydrogen atoms and two oxygen atoms. Isopropyl valerate is a common name of isopropyl pentanoate. It is a five carbon carboxylic acid. The structure of pentanoic acid is shown below.
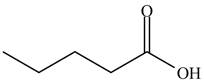
Figure 4
The hydrogen of carboxylic group is replaced by an isopropyl group to form an ester. The structure of isopropyl valerate is shown below.
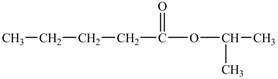
Figure 5
The structure of isopropyl valerate is shown in Figure 5.
(c)
Interpretation:
The structure of ethyl methyl malonate is to be stated.
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
Answer to Problem 21.1P
The structure of ethyl methyl malonate is shown below.
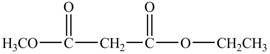
Explanation of Solution
The structure of ethyl methyl malonate consists of a eight carbons, fourteen hydrogen and four oxygen. Ethyl methyl malonate consists of propane dioic acid as parent chain.
The structure of propane dioic acid is shown below.
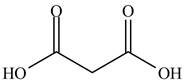
Figure 6
In the above structure, at first position hydrogen is substituted by a methyl group and at the last carboxyl group an ethyl group replaces the hydrogen of the carboxyl group. The structure of ethyl methyl malonate is shown below.
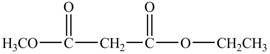
Figure 7
The structure of ethyl methyl malonate is shown in Figure 6.
(d)
Interpretation:
The structure of cyclohexyl acetate is to be stated.
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
Answer to Problem 21.1P
The structure of cyclohexyl acetate is shown below.
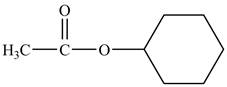
Explanation of Solution
Cyclohexyl acetate consists of a cyclohexane ring and an acetate group. The acetate group is given by the formula
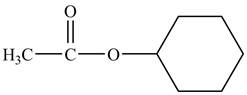
Figure 8
The structure of cyclohexyl acetate is shown in Figure 8
(e)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
Answer to Problem 21.1P
The structure of
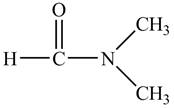
Explanation of Solution
The structure of
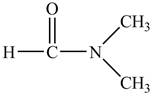
Figure 9
The structure of
(f)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
Answer to Problem 21.1P
The structure of
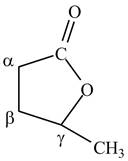
Explanation of Solution
Lactones are cyclic esters. The structure of
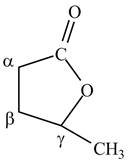
Figure 10
The structure of
(g)
Interpretation:
The structure of glutarimide is to be stated.
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
Answer to Problem 21.1P
The structure of glutarimide is shown below.
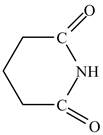
Explanation of Solution
The structure of glutarimide consists of a piperidine ring. There are two carbonyl group present adjacent to the nitrogen group. The structure of a piperidine ring is shown below.
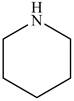
Figure 11
Therefore, the structure of glutarimide is shown below.
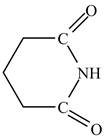
Figure 12
The structure of glutarimide is shown in Figure 12.
(h)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
Answer to Problem 21.1P
The structure of
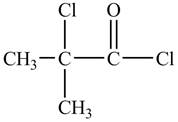
Explanation of Solution
There is carboxyl group present in
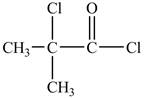
Figure 13
The structure of
(i)
Interpretation:
The structure of
Concept introduction:
Carboxylic acid is a class of organic compound that contains a
Answer to Problem 21.1P
The structure of
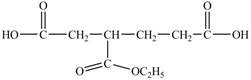
Explanation of Solution
In
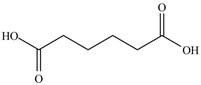
Figure 14
In the above structure there is a ethoxy carbonyl group at the third position. Therefore, the structure of
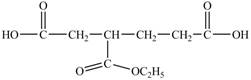
Figure 15
The structure of
Want to see more full solutions like this?
Chapter 21 Solutions
Organic Chemistry
- Using the data in Appendix C, determine which of the following bases is strong enough to deprotonate acetonitrile (CH3CN), so that equilibrium favors the products: (a) NaH; (b) Na2CO3; (c) NaOH; (d) NaNH2; (e) NaHCO3.arrow_forwardDescribe how would you distinguish the following pairs, (a) Benzene and cyclohexane (b) Phenol and toluene (c) Phenol and benzoic acid (d) methanol and isopropyl alcoholarrow_forwardpropanoic acid + methanol (in concentrated sulfuric acid)arrow_forward
- Draw the structures of the following carboxylic acids. (d) cis-4-phenylbut-2-enoic acidarrow_forwardCompounds that contain an N-H group associate by hydrogen bonding. (a) Do you expect this association to be stronger or weaker than that of compounds containing an O-H group? (b) Based on your answer to part (a), which would you predict to have the higher boiling point, 1-butanol or 1-butanamine?arrow_forwardWrite the products of the following acid-base reactions: (a) CH3OH + H2SO4 ² ? (b) CH3OH + NANH2 2 ? (c) CH3NH3+ Cl- + NaOH ?arrow_forward
- Predict the products (if any) of the following acid–base reactions.(a) acetic acid + ammoniaarrow_forward(a) Explain how NaBH, in CH;OH can reduce hemiacetal A to 1,4-butanediol (HOCH,CH,CH,CH,OH). (b) What product is formed when A is treated with Ph;P=CHCH,CH(CH),? (c) The drug isotretinoin is formed by reaction of X and Y. What is the structure of isotretinoin? Although isotretinoin (trade name Accutane or Roaccutane) is used for the treatment of severe acne, it is dispensed under strict controls because it also causes birth defects. PPha NaOCH,CH3 HO- isotretinoin HO A Br X Yarrow_forward1) The carbon-oxygen double bond present in aldehydes and ketones is very polar. What does this mean and how does it arise? 2) The carbon-oxygen double bond is readily attacked by nucleophiles like cyanide ions or ammonia. (i) What do you understand by the term nucleophile? (ii) Which part of the carbon-oxygen double bond is attractive to nucleophiles? 3) Why is there a difference between aldehydes and ketones in their response to oxidizing agents such as potassium dichromate(VI) solution acidified with dilute sulfuric acid?arrow_forward
- Treating choline with acetic anhydride gives acetylcholine, a neurotransmitter. Write an equation for the formation of acetylcholine. (CH,),NCH,CH,OH Cholinearrow_forwardGive a chemical test to distinguish between each of the following pairs of compounds :(i) Ethylamine and Aniline(ii) Aniline and Benzylaminearrow_forwardDraw the structures of the following compounds.(a) ethanoic acidarrow_forward
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
