
Concept explainers
(a)
Interpretation:
The structure of N-isopropylaniline is to be drawn.
Concept introduction:
Answer to Problem 23.1P
The structure of N-isopropylaniline is shown below.
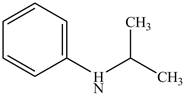
Explanation of Solution
The name N-isopropylaniline indicates that it consists of the structure of aniline compound. One hydrogen atom of
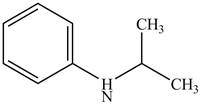
Figure 1
The structure of N-isopropylaniline is shown in Figure 1.
(b)
Interpretation:
The structure of tert-butylamine is to be drawn.
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia. Amines are basic in nature because the nitrogen can donate its lone pairs and also the ability of the nitrogen to accept the proton in water.
Answer to Problem 23.1P
The structure of tert-butylamine is shown below.
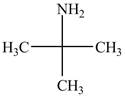
Explanation of Solution
The name tert-butylamine indicates that it consist of tert-butyl group. The tert-butyl group is attached to an amine group. The structure of tert-butylamine is shown below.
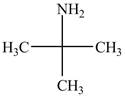
Figure 2
The structure of tert-butylamine is shown in Figure 2.
(c)
Interpretation:
The structure of
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia. Amines are basic in nature because the nitrogen can donate its lone pairs and also the ability of the nitrogen to accept the proton in water.
Answer to Problem 23.1P
The structure of
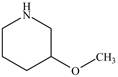
Explanation of Solution
The name
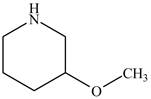
Figure 3
The structure of
(d)
Interpretation:
The structure of
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia. Amines are basic in nature because the nitrogen can donate its lone pairs and also the ability of the nitrogen to accept the proton in water.
Answer to Problem 23.1P
The structure of
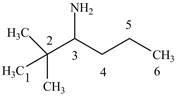
Explanation of Solution
The name
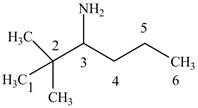
Figure 4
The structure of
(e)
Interpretation:
The structure of
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia. Amines are basic in nature because the nitrogen can donate its lone pairs and also the ability of the nitrogen to accept the proton in water.
Answer to Problem 23.1P
The structure of
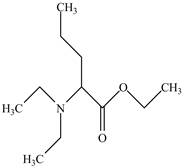
Explanation of Solution
The name
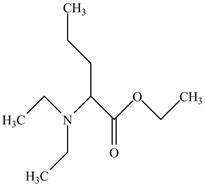
Figure 5
The structure of
(f)
Interpretation:
The structure of
Concept introduction:
Amines are the organic compounds that are formed by replacement of hydrogen from ammonia. Amines are basic in nature because the nitrogen can donate its lone pairs and also the ability of the nitrogen to accept the proton in water.
Answer to Problem 23.1P
The structure of
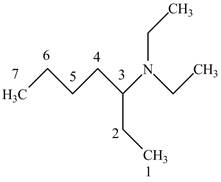
Explanation of Solution
The name
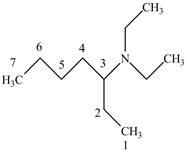
Figure 6
The structure of
Want to see more full solutions like this?
Chapter 23 Solutions
Organic Chemistry
- Draw the structural formulas of the following compounds:(a) 2,3-Dimethylpentanal(b) 1,3-Dibromopropanone(c) 4-hydroxy-4-methylhexan-2-onearrow_forwardWhich of the following is an acceptable name for the compound shown below? (A) meta-ethoxyacetophenone (B) meta-ethoxybenzaldehyde(C) meta-formylanisole (D) meta-ethylacetophenonearrow_forwardDraw a structural formula for each amine. (a) 2-Butanamine (b) 1-Octanamine (c)2,2-Dimethyl-1-propanaminearrow_forward
- From the given structures which is(a) amide that will release a secondary amine upon hydrolysis? (b) product of hydrolysis of MSO (c) a tertiary amide and (d) a diketonearrow_forwardDraw the structure of each of the following molecules. (a) 5-phenylpentanamide; (b) (2S,3S)-2,3-dimethoxyhexanediamide; (c) N-phenylcyclobutanecarboxamidearrow_forwardDescribe concisely a chemical test to distinguish between the following pairs of compounds.(a) Propanal and propanone(b) Phenol and benzoic acid(c) Hexan-3-one and hexan-2-onearrow_forward
- Draw the structure of the product that will be formed when each of the following amines reacts with sodium nitrite and hydrochloric acid, followed by cuprous chloride. (a) propylamine (b) dipropylamine (c) N-propylaniline (d) N,N-dipropylaniline (e) p-propylanilinearrow_forwardPredict the major products formed when the following amines undergo exhaustivemethylation, treatment with Ag2O, and heating.(a) hexan-2-aminearrow_forward1. Draw structures corresponding to the following IUPAC names: (a) 4-Methylpentanoic acid (b) o-Hydroxybenzoic acid (c) 2,2-Dimethylpropanoyl chloride (d) trans-2-Methylcyclohexanecarboxamide (e) p-Methylbenzoic anhydride (f) p-Bromobenzonitrilearrow_forward
- 14. What is the IUPAC name for the following compound? NH- (a) propyl propanamide (b) propyl propanoate (c) N-propylptopanamine. (d) N, N-propylpropanamide (e) N-propxlpropanamidearrow_forwardDraw a structural formula for each amine and amine derivative. (a) N,N-Dimethylaniline (b) Triethylamine (c) tert-Butylamine (d) 1,4-Benzenediamine (e) 4-Aminobutanoic acid (f) (R)-2-Butanamine (g) Benzylamine (h) trans-2-Aminocyclohexanol (i) 1-Phenyl-2-propanamine (amphetamine) (j) Lithium diisopropylamide (LDA) (k) Benzyltrimethylammonium hydroxide (Triton B)arrow_forwardDraw the structure of the product that will be formed when each of the following amines reacts with sodium nitrite and hydrochloric acid, followed by cuprous chloride. (d) N,N-dipropylanilinearrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





