
Organic Chemistry As a Second Language: Second Semester Topics
4th Edition
ISBN: 9781119110651
Author: David R. Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 2.5, Problem 2.15P
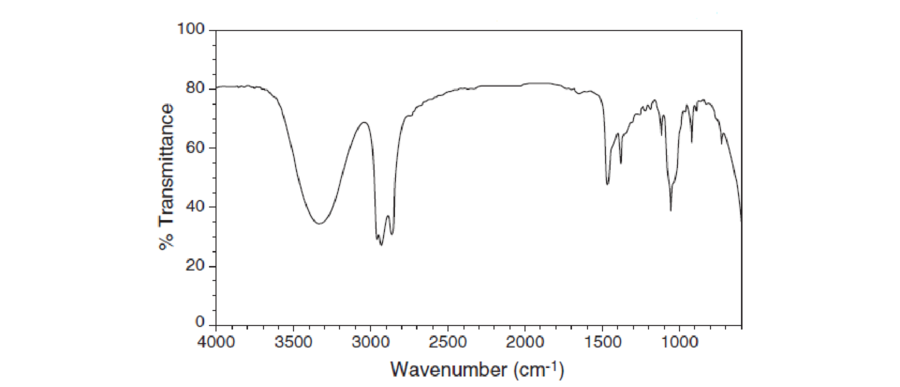
For each IR spectrum below, determine whether it is consistent with the structure of a
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Analyse the high resolution proton NMR spectrum of a compound with a molecular formula of C8H16O2 and and write its name.
A) 2-ethylhexanoic acid
B) 1,4-cyclohexanedimethanol
C) ethyl hexanoate
D) butyl butyrate
E) ethyl 2,2-dimethylpropanoate
The DEPT-90 spectrum exhibits
6
in the 0-50 ppm region
The DEPT-135 spectrum exhibits
x
100 ppm region that is a
positive ▾
C6
signal(s) for the CH groups:
▼
1,2,6
✓
in the sp2 hybridized region 100-150
C3 and C4 ▼
signal(s) (only the quaternary carbon atoms,
signal(s), indicating the presence of a methylene group (CH₂) attached to an oxygen atom,
are missing); there is
C5
▼
C1 and C2 ▼
and
signal(s) in the 50-
don't use handwriting
1.
MORONS
20
The IR spectrum above indicates the presence of which of the following bond
types or functional groups in the molecule? Select two.
100
90
70
40
4000
3000
Wavenumber (em')
2000
1000
The IR spectrum above indicates the presence of which of the following bond
types or functional groups in the molecule? Select two.
select two for each picture
alcohol
alkyne
aldehyde or ketone carbonyl
primary amine
secondary amine
carboxylić acid
nitrile
sp2 C-H
Chapter 2 Solutions
Organic Chemistry As a Second Language: Second Semester Topics
Ch. 2.3 - For each of the following compounds, determine...Ch. 2.3 - For each of the following compounds, determine...Ch. 2.3 - For each of the following compounds, determine...Ch. 2.3 - For each of the following compounds, determine...Ch. 2.3 - The following compound has three carbonyl groups....Ch. 2.4 - Predict which of the following C=C bonds will...Ch. 2.4 - The C=C bond in the following compound produces an...Ch. 2.5 - For each IR spectrum below, identify whether it is...Ch. 2.5 - For each IR spectrum below, identify whether it is...Ch. 2.5 - For each IR spectrum below, identify whether it is...
Ch. 2.5 - For each IR spectrum below, identify whether it is...Ch. 2.5 - For each IR spectrum below, identify whether it is...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.5 - For each IR spectrum below, determine whether it...Ch. 2.6 - Prob. 2.22P
Additional Science Textbook Solutions
Find more solutions based on key concepts
69 (a) What is the difference between a CFC and an HFC? (b) It is estimated that the lifetime for HFCs in the s...
Chemistry: The Central Science (13th Edition)
Draw the mechanism for the reaction of cyclohexene with HCl.
Organic Chemistry
For each of the following, (i) give the systematic name of the compound and specify the oxidation state of the ...
General Chemistry: Atoms First
Why isn't FeBr3 used as a catalyst in the first step of the synthesis of 1,3,5-tribromobenzene?
Organic Chemistry (8th Edition)
Which of the following species would you expect to be diamagnetic and which paramagnetic? (a) Ka; (b) Cr24 (c) ...
General Chemistry: Principles and Modern Applications (11th Edition)
9.1 Calculate the total mass of the reactants and the products for each of the following equations:
Basic Chemistry (5th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Which of the following functional group can be predicted with the IR spectrum shown? 2150 cm1 3320 cm 2950 cm1 4000 3500 3000 2500 2000 1500 1000 Ketone carboxylic acid Alkyne Primary amine Alkenearrow_forwardDraw structures for a saturated hydrocarbon that has a molecular ion with an m/z value of 128.arrow_forwardWhat functional group is indicated by the IR spectrum below? A) Aldehyde B) Ketone C) Carboxylic Acid D) Alkene E) Aminearrow_forward
- 4. Give the structure of each of the following compounds. a) a six-carbon alkane whose proton NMR consists of 1 singlet. b) a six-carbon alkene whose proton NMR consists of 1 singlet c) a nine-carbon alkane whose proton nmr consists of 2 singlets.arrow_forwardAn IR spectrum of an unknown compound is shown below. Predict the principal functional group present in this compound. 4000 3000 2000 1500 Wavenumber (cm-¹) 1000 500 A) amide B) amine C) carboxylic acid D) ether E) aldehydearrow_forwardHow many signals will the following compound display in the proton NMR spectrum? Compound: CH3CH,CH2COOCH,CH(CH3)2 O 6 O 7 3 O 5 4arrow_forward
- 3. Which of the following information is primarily obtained from infrared spectroscopy? A) arrangement of carbon and hydrogen atoms in a compound B) molecular weight of a compound C) any conjugated t system present in a compound D) functional groups present in a compound E) all of thesearrow_forwardWhat is this compound base on the IR spectrum?arrow_forwarddraw the strucutre based off the IR spectrum, 1H NMR spectrum, and 13C NMR spectrum for this compound. This compound is an ester that is commonly used as food additive to give foods and beverages a fruity flavor. Its molecular formula is C7H14O2arrow_forward
- 2. Draw structures for compounds that have the proton nmrs described: a) C2H6O; one singlet b) CAH&Cl2O; two triplets c) CAHBO2; one singlet, one triplet and one quartetarrow_forwardPlease solve this organic chemistry question A carboxylic acid with the molecular formula C5H10O2 is treated with thionyl chloride to give compound A. Compound A has only one signal in its 1H NMR spectrum. Please figure out this carboxylic acid. You can write out name directly or molecular formula with functional groups (e.g. isopropanol (CH3)3COH). Hint: Only one H NMR signal means all hydrogens are in the same chemical status.arrow_forward1H NMR spectrum is shown below. Is it an ester found in wintergreen oil or an ester that is commonly used as a pain reliever. Both esters can be made from salicylic acid. Based on this information, determine the structure/identity of the ester corresponding to Spectrum 1.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Mass Spectrometry; Author: Professor Dave Explains;https://www.youtube.com/watch?v=hSirWciIvSg;License: Standard YouTube License, CC-BY