
Concept explainers
(a)
Interpretation:
The explanation for alanine which has different optical rotation in water,
Concept introduction:
The amino acid is made of two
Answer to Problem 27.61AP
Each solvent rotates the alanine molecule to a different angle due to the formation of different complex formation and so it gives rise to different optical rotation in different solvents.
Explanation of Solution
The structure of alanine is shown below.
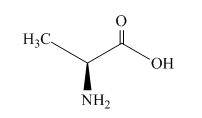
Figure 1
Alanine is an optically active compound. It rotates the plane of polarized light. The reaction of alanine with water,
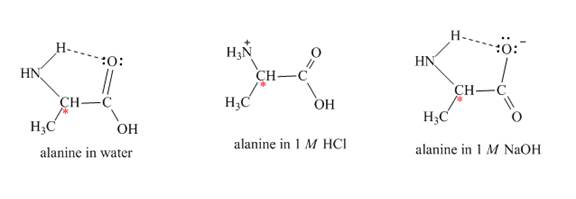
Figure 2
The optical rotation of alanine is measure by Circular Dichroism (CD). It involves circularly polarized light absorption. It measures the angle at which the plane-polarized light is rotated by the molecule. Each solvent rotates the alanine molecule to a different angle, due to this it gives rise to different optical rotation in different solvents.
The alanine has different optical rotation in water,
(b)
Interpretation:
The explanation for two known mono
Concept introduction:
The amino acid is made of two functional groups an amine group,
Answer to Problem 27.61AP
Due to the presence of two amine groups in lysine molecule. It may undergo acetylation reaction from either side and form mono
Explanation of Solution
The structure of amino acid lysine is shown below.
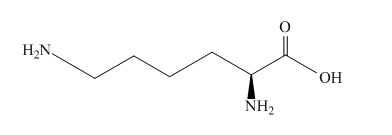
Figure 3
The structure of the lysine molecule contains two amine group one at the
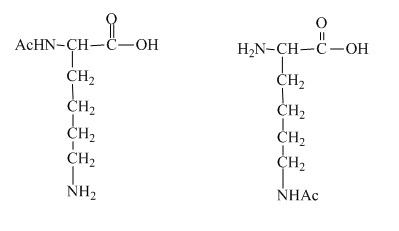
Figure 4
The two mono
(c)
Interpretation:
The explanation for fact that the peptide
Concept introduction:
The amino acid is made of two functional groups an amine group
Answer to Problem 27.61AP
The compound urea under basic conditions acts as a denaturation agent which breakdown the protein molecule bonding. Due to this, the peptide,
Explanation of Solution
The protein molecule is composed of four types of structure primary, secondary, tertiary and quarternary. The enzyme trypsin hydrolyzes the peptide,
The peptide
(d)
Interpretation:
The explanation for the peptide containing cysteine on reaction with
Concept introduction:
The amino acid is made of two functional groups an amine group,
Answer to Problem 27.61AP
The generation of lysine type molecule at the end of the reaction which is cleaved by trypsin enzyme. Due to this, the peptide containing cysteine on reaction with
Explanation of Solution
The structure of the cysteine molecule is shown below.
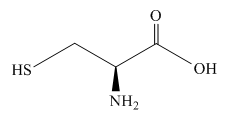
Figure 5
The same molecule in peptides exists as a disulfide bond. The disulfide bond of cysteine in the protein molecule is shown below.
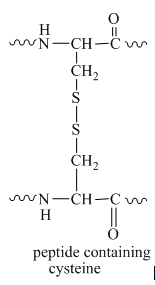
Figure 6
When this protein molecule with a disulfide bond is treated with
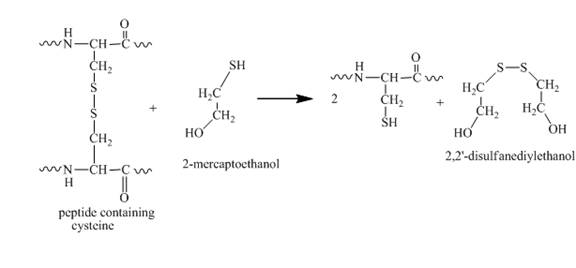
Figure 7
The thiol group is then reacted with the aziridine molecule which results in the formation of a lysine type molecule. This reaction is shown below.
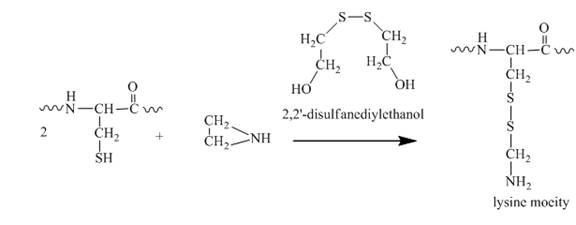
Figure 8
This lysine type molecule then reacts with trypsin enzyme which cleaves arginine and lysine molecule. Due to this, the trypsin enzyme reacts with the modified cysteine residues.
The peptide containing cysteine on reaction with
(e)
Interpretation:
The explanation for the formation of two separable methionine sulfoxides from the oxidation of
Concept introduction:
The amino acid is made of two functional groups an amine group,
Answer to Problem 27.61AP
The application of a certain amount of energy which converts one form to another form of structure. Due to this, the formation of two separable methionine sulfoxides from the oxidation of
Explanation of Solution
The structure of methionine is shown below.
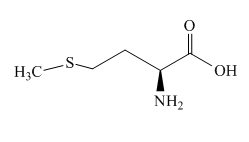
Figure 9
The resonance structure of sulfoxides with two different groups is shown below.
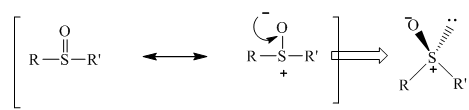
Figure 10
The conversion of one structure to another structure at room temperature requires a certain amount of energy. Therefore, on the application of that amount of energy, the two forms of
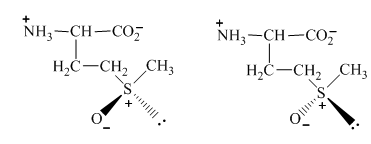
Figure 11
The formation of two separable methionine sulfoxides from the oxidation of
Want to see more full solutions like this?
Chapter 27 Solutions
Organic Chemistry
- Indicate whether each statement is true or false. (a) Tryptophan is an aromatic amino acid. (b) Lysine is positively charged at pH 7. (c) Asparagine has two amide bonds. (d) Isoleucine and leucine are enantiomers. (e) Valine is probably more water-soluble than arginine.arrow_forwardThe amino acids in which the R groups have a net positive charge at pH 7.0 are (A) Lysine, Arginine, Histidine (B) Lysine, Aspargine (c) Histidine, Aspargine (D) Glutamine, Argininearrow_forwardAn enzyme catalyzes the hydrolysis of an ester with a certain activity, but this activity is lost in a 3 M urea solution. What is the most likely explanation for the loss of activity? (A) Urea binds to the active site of the enzyme competitively with the substrate. (B) Urea causes the cleavage of the peptide bonds in the enzyme. (C) Urea causes the enzyme to denature and lose its specific three-dimensional shape. (D) Urea reacts with disulfide bonds in the enzyme.arrow_forward
- The peptide Proline-Serine-Alanine-Phenylalanine-Glutamine is present at pH 7. Draw the peptide and include stereochemistry.arrow_forwardGive the amino acid sequence of an octapeptide that contains the amino acids Tyr, Ala, Leu (2 equiv), Cys, Gly, Glu, and Val, and forms the following fragments when partially hydrolyzed with HCl: Val–Cys–Gly–Glu, Ala–Leu–Tyr, and Tyr–Leu–Val–Cys.arrow_forward11. The structures of three amino acids are given below : NH2 0 Но -CH,CH-C-OH HOOCCH2CH2CH(NH2)COOH (1) (II)arrow_forward
- Write the complete structures for the following peptides. Tell whether each peptide is acidic, basic, or neutral.(a) methionylthreonine (b) threonylmethioninearrow_forwardDraw the amino acids and peptide fragments formed when the decapeptide A–P–F–L–K–W–S–G–R–G is treated with each reagent or enzyme: (a) chymotrypsin; 8 pt helvetica roman (b) trypsin; (c) carboxypeptidase; (d) C6H5N = C = S.arrow_forwardThe use of salt bridges or hydrophobic interactions (or pockets) to stabilize interactions between more distant amino acids within a single polypeptide, is a demonstration of protein TERTIARY structure. True Falsearrow_forward
- 2. (a) Describe any two of the secondary structure elements found in peptides. (b) Why do Glycine and Proline destabilise an a-helix secondary structure element? (c) Provide a definition of the term isoelectric point? (d) Using the information given below, calculate the pl of the amino acid citrulline. H₂N pK₂ = 9.23 H₂N. NH COOH pK₂ = 2.27arrow_forwardWrite the structure of all possible peptides containing these amino acids: Ala, Leu, Use single letter abbreviations and capital letters only; i.e. GYR, not Gly-Tyr-Arg. If there are fewer than 6 peptides, leave an appropriate number of answer boxes empty.arrow_forwardindicate the RIGHT alternative: (a) The Zwitterion form of an amino acid exists only at a point pH value. (b) In a peptide bond there is free rotation at the C-N bond. (c) In a polypeptide, the terminal carboxyl group may be present in its amide form. (d) At a pH greater than pI, an amino acid tends to move towards the cathode in an electrophoresis. (e) At any pH below pI, the predominant form of an amino acid is negatively charged.arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning

