
Organic Chemistry - Standalone book
10th Edition
ISBN: 9780073511214
Author: Francis A Carey Dr., Robert M. Giuliano
Publisher: McGraw-Hill Education
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 3.15, Problem 19P
Interpretation Introduction
Interpretation:
The most stable conformation of the piperidine derivative is to be drawn. In piperidine derivative the hydrogen bonded to nitrogen has been replaced by methyl.
Concept introduction:
The position of axial and equatorial bonds in cyclohexane chair form is
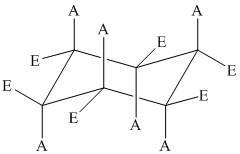
A substituent at an equatorial position is more stable than a substituent at an axial position.
A methyl group has
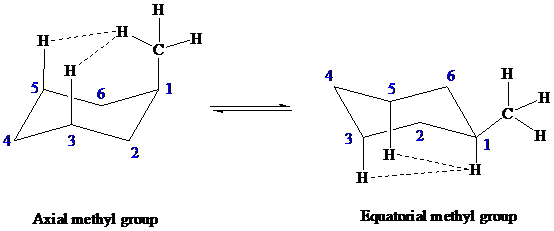
The van der Waals strain is greater between the axial hydrogen and the hydrogen of axial methyl group at
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Unlike most hydrates, the hydrate of cyclopropanone is stable and can be isolated. Explain why this hydrate is stable.
Name each of the following cyclic sugars. (Consult Fig. 5-32 on p. 249 for the name of each sugar in its acyclic form.)
(a)
OH
H
(b)
OH
(c)
Но
НО
OH
Но
Но
Но
H
HỌ
HO-
H.
-H
H
ОН
н но
OH
H
OH
H
Нонн
What will be the optical conformation of the product in the following reaction?
Chapter 3 Solutions
Organic Chemistry - Standalone book
Ch. 3.1 - Identify the alkanes corresponding to each of the...Ch. 3.1 - Find the conformations in Figure 3.4 in which the...Ch. 3.2 - Sketch a potential energy diagram for rotation...Ch. 3.2 - Acetylcholine is a neurotransmitter in the central...Ch. 3.2 - Prob. 5PCh. 3.5 - The heats of combustion of ethylcyclopropane and...Ch. 3.8 - Prob. 7PCh. 3.10 - The following questions relate to a cyclohexane...Ch. 3.10 - Draw the most stable conformation of...Ch. 3.11 - Prob. 10P
Ch. 3.11 - Prob. 11PCh. 3.12 - Based on what you know about disubstituted...Ch. 3.12 - Write structural formulas for the most stable...Ch. 3.14 - Cubane (C4H8) is the common name of the polycyclic...Ch. 3.14 - Prob. 15PCh. 3.14 - Prob. 16PCh. 3.14 - Prob. 17PCh. 3.14 - Prob. 18PCh. 3.15 - Prob. 19PCh. 3 - Give the IUPAC names of each of the following: (a)...Ch. 3 - Draw Newman projections for the gauche and...Ch. 3 - Identify all atoms that are (a) anti and (b)...Ch. 3 - Prob. 23PCh. 3 - Prob. 24PCh. 3 - Prob. 25PCh. 3 - Prob. 26PCh. 3 - Prob. 27PCh. 3 - Prob. 28PCh. 3 - Oxidation of 4-tert-butylthiane proceeds according...Ch. 3 - The following are representations of two forms of...Ch. 3 - Draw (a) a Newman projection of the most stable...Ch. 3 - Write a structural formula for the most stable...Ch. 3 - Sight down the C-2-C-3 bond, and draw Newman...Ch. 3 - Prob. 34PCh. 3 - Sketch an approximate potential energy diagram for...Ch. 3 - Prob. 36PCh. 3 - Even though the methyl group occupies an...Ch. 3 - Which do you expect to be the more stable...Ch. 3 - Arrange the trimethylcyclohexane isomers shown in...Ch. 3 - Identify the more stable stereoisomer in each of...Ch. 3 - One stereoisomer of 1,1,3,5-tetramethylcyclohexane...Ch. 3 - One of the following two stereoisomers is...Ch. 3 - In each of the following groups of compounds,...Ch. 3 - The heats of combustion of the more and less...Ch. 3 - The measured dipole moment of ClCH2CH2Cl is 1.12D....Ch. 3 - Prob. 46PCh. 3 - Prob. 47PCh. 3 - Prob. 48DSPCh. 3 - Prob. 49DSPCh. 3 - Prob. 50DSPCh. 3 - Prob. 51DSPCh. 3 - Prob. 52DSPCh. 3 - Prob. 53DSP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- What type of base should you use?Draw in Newman projection the reactive conformationWhat will be the major product of this reaction? Give the driving mechanism and indicate the stereospecificity using a clear three-dimensional topological structurearrow_forwardWhich of the following are achiral? And identify the relationship between each of them. Ex. A-B, B-Darrow_forwardWhen we have discussed the enol-keto tautomerization of ketones in class, the isomerization has been facililated by an acid or base catalyst, with most ketones heavily favoring the carbonyl isomer at equilibrium. However, samples of the ketone below in its pure form (with no acid, base or water present) contain nearly 10% of the enol form! OH -90% -10% From this data, we can infer that the enol isomer of this ketone is particularly stable. Explain the stability of this enol, using terms and ideas that we have discussed in this unit.arrow_forward
- Resveratrol is an antioxidant found in the skin of red grapes. Its anticancer, anti-inammatory, and various cardiovascular effects are under active investigation. (a) Draw all resonance structures for the radical that results from homolysis of the OH bond shown in red. (b) Explain why homolysis of this OH bond is preferred to homolysis of either OH bond in the other benzene ring.arrow_forwardGiven that syn addition of H2 occurs from both sides of a trigonal planar double bond, draw all stereoisomers formed when attached compound is treated with H2.arrow_forwardResveratrol is an antioxidant found in the skin of red grapes. Itsanticancer, anti-inflammatory, and various cardiovascular effects areunder active investigation. (a) Draw all resonance structures for theradical that results from homolysis of the OH bond shown in red. (b)Explain why homolysis of this OH bond is preferred to homolysis ofeither OH bond in the other benzene ring.arrow_forward
- What carbon radical is formed by homolysis of the C–Ha bond inpropylbenzene? Draw all reasonable resonance structures for thisradical.arrow_forwardEach of the following line structures can be described as a bicyclononane except for.arrow_forwardIn base-catalyzed halogenation of acetone, the second (and third) on the same carbon. How do you explain why while halogenation takes place, it does not occur on the carbon in the other methyl group?arrow_forward
- Which of the following is capable of producing longer alkyne products? (A) Internal alkyne with NaNH2 and Alkyl halides B Terminal alkyne with NANH2 and Alkyl halides Internal alkene with NANH2 and Alkyl halides Terminal alkene with NANH2 and Alkyl halidesarrow_forwardWhich of these isomers of trimethylbenzene will produce exclusively one monobrominated product when treated with Br2 and FeBr3? Explain.arrow_forwardFor each of the following molecules draw the most stable conformations indicated in the parenthesis, and along the specified bonds.a) sec-butylcyclohexane (Newman projection chair along C1-C2 and C5-C4)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License