
Concept explainers
(a)
Interpretation:
Assign the correct shape / geometry to 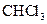 (chloroform), based on VSEPR theory.
(chloroform), based on VSEPR theory.
Concept introduction:
According to VSEPR theory we can determine the shape of a molecule by following the given steps
- First identify the number of bonded atoms to the central atom and count the number of lone pair of electrons on central atom. Add these.
- The sum obtained above gives us idea about the electronic geometry in a molecule. For example if it is two then the electron geometry will be linear, if it is three the geometry will be trigonal planar, four the geometry will be tetrahedral, five the geometry will be trigonal bipyramidal, six the geometry will be octahedral.
- Now for molecular geometry we have to consider the number of lone pair of electrons.
- The molecular geometry depends upon the repulsion order between electron pairs which is Bond pair-bond pair < lone pair −bond pair < lone pair-lone pair.
(b)
Interpretation:
Assign the correct shape / geometry to 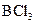 (boron trichloride), based on VSEPR theory.
(boron trichloride), based on VSEPR theory.
Concept introduction:
According to VSEPR theory we can determine the shape of a molecule by following the given steps
- First identify the number of bonded atoms to the central atom and count the number of lone pair of electrons on central atom. Add these.
- The sum obtained above gives us idea about the electronic geometry in a molecule. For example if it is two then the electron geometry will be linear, if it is three the geometry will be trigonal planar, four the geometry will be tetrahedral, five the geometry will be trigonal bipyramidal, six the geometry will be octahedral.
- Now for molecular geometry we have to consider the number of lone pair of electrons.
- The molecular geometry depends upon the repulsion order between electron pairs which is Bond pair -bond pair < lone pair −bond pair < lone pair-lone pair.
(c)
Interpretation:
Assign the correct shape / geometry to 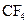 (carbon tetrafluoride), based on VSEPR theory.
(carbon tetrafluoride), based on VSEPR theory.
Concept introduction:
According to VSEPR theory we can determine the shape of a molecule by following the given steps
- First identify the number of bonded atoms to the central atom and count the number of lone pair of electrons on central atom. Add these.
- The sum obtained above gives us idea about the electronic geometry in a molecule. For example if it is two then the electron geometry will be linear, if it is three the geometry will be trigonal planar, four the geometry will be tetrahedral, five the geometry will be trigonal bipyramidal, six the geometry will be octahedral.
- Now for molecular geometry we have to consider the number of lone pair of electrons.
- The molecular geometry depends upon the repulsion order between electron pairs which is Bond pair--bond pair< lone pair −bond pair < lone pair-lone pair.
(d)
Interpretation:
Assign the correct shape / geometry to 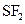 (sulfur difluoride), based on VSEPR theory.
(sulfur difluoride), based on VSEPR theory.
Concept introduction:
According to VSEPR theory we can determine the shape of a molecule by following the given steps
- First identify the number of bonded atoms to the central atom and count the number of lone pair of electrons on central atom. Add these.
- The sum obtained above gives us idea about the electronic geometry in a molecule. For example if it is two then the electron geometry will be linear, if it is three the geometry will be trigonal planar, four the geometry will be tetrahedral, five the geometry will be trigonal bipyramidal, six the geometry will be octahedral.
- Now for molecular geometry we have to consider the number of lone pair of electrons.
- The molecular geometry depends upon the repulsion order between electron pairs which is Bond pair-bond pair < lone pair −bond pair < lone pair-lone pair.
(e)
Interpretation:
Assign the correct shape / geometry to 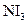 (Nitrogen triiodide), based on VSEPR theory.
(Nitrogen triiodide), based on VSEPR theory.
Concept introduction:
According to VSEPR theory we can determine the shape of a molecule by following the given steps
- First identify the number of bonded atoms to the central atom and count the number of lone pair of electrons on central atom. Add these.
- The sum obtained above gives us idea about the electronic geometry in a molecule. For example if it is two then the electron geometry will be linear, if it is three the geometry will be trigonal planar, four the geometry will be tetrahedral, five the geometry will be trigonal bipyramidal, six the geometry will be octahedral.
- Now for molecular geometry we have to consider the number of lone pair of electrons.
- The molecular geometry depends upon the repulsion order between electron pairs which is Bond pair-bond pair < lone pair −bond pair < lone pair-lone pair.
(f)
Interpretation:
Assign the correct shape / geometry to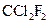 (Dichlorodifluoromethane), based on VSEPR theory.
(Dichlorodifluoromethane), based on VSEPR theory.
Concept introduction:
According to VSEPR theory we can determine the shape of a molecule by following the given steps
- First identify the number of bonded atoms to the central atom and count the number of lone pair of electrons on central atom. Add these.
- The sum obtained above gives us idea about the electronic geometry in a molecule. For example if it is two then the electron geometry will be linear, if it is three the geometry will be trigonal planar, four the geometry will be tetrahedral, five the geometry will be trigonal bipyramidal, six the geometry will be octahedral.
- Now for molecular geometry we have to consider the number of lone pair of electrons.
- The molecular geometry depends upon the repulsion order between electron pairs which is Bond pair-bond pair < lone pair −bond pair < lone pair-lone pair.
Want to see the full answer?
Check out a sample textbook solution
Chapter 4 Solutions
Chemistry For Changing Times (14th Edition)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





