
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
8th Edition
ISBN: 9781305387102
Author: Kreith, Frank; Manglik, Raj M.
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 1, Problem 1.63P
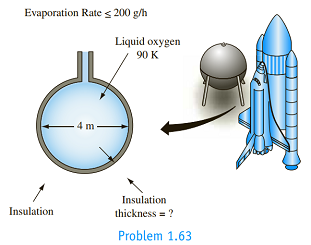
Liquid oxygen (LOX) for the space shuttle is stored at 90 K prior to launch in a spherical container 4 m in diameter. To reduce the loss of oxygen, the sphere is insulated with superinsulation developed at the U.S. National Institute of Standards and Technology's Cryogenic Division; the superinsulation has an effective thermal conductivity of 0.00012 W/m K. If the outside temperature is
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
The boiling temperature of nitrogen at atmospheric pressure at sea level (1 atm) is -196°C. Therefore, nitrogen is commonly used in low temperature scientific studies since the temperature of liquid nitrogen in a tank open to the atmosphere will remain constant at -196°C until the liquid nitrogen in the tank is depleted. Any heat transfer to the tank will result in the evaporation of some liquid nitrogen, which has a heat of vaporization of 198 kJ/kg and a density of 810 kg/m3 at 1 atm. Consider a 3-m-diameter spherical tank initially filled with liquid nitrogen at 1 atm and 196°C. The tank is exposed to 22°C ambient air with a heat transfer coefficient of 22 W/m2 · °C. The temperature of the thin-shelled spherical tank is observed to be almost the same as the temperature of the nitrogen inside. Disregarding any radiation heat exchange, determine the rate of evaporation of the liquid nitrogen in the tank as a result of the heat transfer from the ambient air in kg/sec. Answer in…
A father and son conducted the following simple experiment on a hot dog which measured 12.5 cm in length and 2.2 cm in diameter. They inserted one food thermometer into the midpoint of the hot dog and another one was placed just under the skin of the hot dog. The temperatures of the thermometers were monitored until both thermometers read 20°C, which is the ambient temperature. The hot dog was then placed in 94°C boiling water and after exactly 2 min they recorded the center temperature and the skin temperature of the hot dog to be 59°C and 88°C, respectively. Assuming the following properties for the hot dog: r = 980 kg/m3 and cp = 3900 J/kg·K and using transient temperature charts, determine (a) the thermal diffusivity of the hot dog, (b) the thermal conductivity of the hot dog, and (c) the convection heat transfer coefficient.
The author and his then 6-year-old son have conducted the following experiment to determine the thermal conductivity of a hot dog. They first boiled water in a large pan and measured the temperature of the boiling water to be 94°C, which is not surprising, since they live at an elevation of about 1650 m in Reno, Nevada. They then took a hot dog that is 12.5 cm long and 2.2 cm in diameter and inserted a thermocouple into the midpoint of the hot dog and another thermocouple just under the skin. They waited until both thermocouples read 20°C, which is the ambient temperature. They then dropped the hot dog into boiling water and observed the changes in both temperatures. Exactly 2 min after the hot dog was dropped into the boiling water, they recorded the center and the surface temperatures to be 59°C and 88°C, respectively. The density of the hot dog can be taken to be 980 kg/m3, which is slightly less than the density of water, since the hot dog was observed to be floating in water while…
Chapter 1 Solutions
Principles of Heat Transfer (Activate Learning with these NEW titles from Engineering!)
Ch. 1 - 1.1 On a cold winter day, the outer surface of a...Ch. 1 - 1.2 The weight of the insulation in a spacecraft...Ch. 1 - 1.3 A furnace wall is to be constructed of brick...Ch. 1 - 1.4 To measure thermal conductivity, two similar...Ch. 1 - To determine the thermal conductivity of a...Ch. 1 - A square silicon chip 7mm7mm in size and 0.5-mm...Ch. 1 - A cooling system is to be designed for a food...Ch. 1 - 1.80 Describe and compare the modes of heat loss...Ch. 1 - Heat is transferred at a rate of 0.1 kW through...Ch. 1 - 1.10 A heat flux meter at the outer (cold) wall of...
Ch. 1 - 1.11 Calculate the heat loss through a glass...Ch. 1 - 1.12 A wall with a thickness is made of a...Ch. 1 - 1.13 If the outer air temperature in Problem is...Ch. 1 - Using Table 1.4 as a guide, prepare a similar...Ch. 1 - 1.15 A thermocouple (0.8-mm-diameter wire) used to...Ch. 1 - Water at a temperature of 77C is to be evaporated...Ch. 1 - The heat transfer rate from hot air by convection...Ch. 1 - The heat transfer coefficient for a gas flowing...Ch. 1 - 1.19 A cryogenic fluid is stored in a...Ch. 1 - A high-speed computer is located in a...Ch. 1 - 1.21 In an experimental set up in a laboratory, a...Ch. 1 - 1.22 In order to prevent frostbite to skiers on...Ch. 1 - Using the information in Problem 1.22, estimate...Ch. 1 - Two large parallel plates with surface conditions...Ch. 1 - 1.25 A spherical vessel, 0.3 m in diameter, is...Ch. 1 - 1.26 Repeat Problem 1.25 but assume that the...Ch. 1 - Determine the rate of radiant heat emission in...Ch. 1 - 1.28 The sun has a radius of and approximates a...Ch. 1 - 1.29 A spherical interplanetary probe with a 30-cm...Ch. 1 - A spherical communications satellite, 2 m in...Ch. 1 - A long wire 0.7 mm in diameter with an emissivity...Ch. 1 - Wearing layers of clothing in cold weather is...Ch. 1 - A section of a composite wall with the dimensions...Ch. 1 - A section of a composite wall with the dimensions...Ch. 1 - Repeat Problem 1.35 but assume that instead of...Ch. 1 - 1.37 Mild steel nails were driven through a solid...Ch. 1 - Prob. 1.38PCh. 1 - 1.39 On a cold winter day, the outside wall of a...Ch. 1 - As a designer working for a major electric...Ch. 1 - 1.41 A heat exchanger wall consists of a copper...Ch. 1 - 1.43 A simple solar heater consists of a flat...Ch. 1 - A composite refrigerator wall is composed of 5 cm...Ch. 1 - An electronic device that internally generates 600...Ch. 1 - 1.47 A flat roof is modeled as a flat plate...Ch. 1 - A horizontal, 3-mm-thick flat-copper plate, 1-m...Ch. 1 - 1.49 A small oven with a surface area of is...Ch. 1 - A steam pipe 200 mm in diameter passes through a...Ch. 1 - 1.51 The inner wall of a rocket motor combustion...Ch. 1 - 1.52 A flat roof of a house absorbs a solar...Ch. 1 - Determine the power requirement of a soldering...Ch. 1 - 1.54 The soldering iron tip in Problem 1.53...Ch. 1 - Prob. 1.55PCh. 1 - A pipe carrying superheated steam in a basement at...Ch. 1 - Draw the thermal circuit for heat transfer through...Ch. 1 - 1.60 Two electric resistance heaters with a 20 cm...Ch. 1 - 1.63 Liquid oxygen (LOX) for the space shuttle is...Ch. 1 - The interior wall of a large, commercial walk-in...Ch. 1 - 1.67 In beauty salons and in homes, a ubiquitous...Ch. 1 - The heat transfer coefficient between a surface...Ch. 1 - The thermal conductivity of fibreglass insulation...Ch. 1 - 1.71 The thermal conductivity of silver at 212°F...Ch. 1 - 1.72 An ice chest (see sketch) is to constructed...Ch. 1 - Estimate the R-values for a 5-cm-thick fiberglass...Ch. 1 - A manufacturer in the United States wants to sell...Ch. 1 - Referring to Problem 1.74, how many kilograms of...Ch. 1 - 1.76 Explain a fundamental characteristic that...Ch. 1 - 1.77 Explain each in your own words. (a) What is...Ch. 1 - What are the important modes of heat transfer for...Ch. 1 - 1.79 Consider the cooling of (a) a personal...Ch. 1 - Describe and compare the modes of heat loss...Ch. 1 - A person wearing a heavy parka is standing in a...Ch. 1 - Discuss the modes of heat transfer that determine...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, mechanical-engineering and related others by exploring similar questions and additional content below.Similar questions
- It is designed in such a way that the internal temperature of a commercial heat treatment furnace can reach up to 165 oC. All surfaces of the furnace consist of firebrick (10 cm), insulation material and sheet metal (3mm) from the inside out. Given that the outdoor temperature is 22 oC, the outer sheet will be allowed to go up to 35 oC, which is a temperature that will not be disturbed by hand contact. In this case, determine the insulation material thickness to be used. Insulation material thermal conductivity coefficient is 0.066 insulation W / m oC, 60 W / m oC for sheet metal and 115 W / m oC for firebrick. Indoor heat transfer coefficient will be accepted as 25 W / m2 oC and 12 W / m2 oC for outdoor environment.arrow_forwarda. What is the heat flux, q"1 [in W/m2], at the left-hand side of layer B? Express your answer as a negative number if the heat flux goes to the left, and as a positive number if the heat flux goes to the right. b.What is the heat flux, q"2 ( in W/m2) at the right-hand side of layer B? Express your answer as a negative number if the heat flux goes to the left, and as a positive number if the heat flux goes to the right. c. What is the temperature, T1, on the left-hand side of layer B, in Celsius? d. What is the temperature, T2, on the right-hand side of layer B, in Celsius?arrow_forwardIt is designed in such a way that the internal temperature of a commercial heat treatment furnace can reach up to 165 oC. All surfaces of the furnace consist of firebrick (10 cm), insulation material and sheet metal (3mm) from the inside out. Given that the outdoor temperature is 22 oC, the outer sheet will be allowed to go up to 35 oC, which is a temperature that will not disturb in contact with hands. In this case, determine the insulation material thickness to be used. The thermal conductivity coefficient of the insulation material is insulation 0.066 W / m oC, 60 W / m oC for sheet metal and 115 W / m oC for firebrick. Indoor heat transfer coefficient will be accepted as 25 W / m2 oC and 12 W / m2 oC for outdoor environment.arrow_forward
- A cooking pan is made of aluminium of mass 2kg and a specific heat capacity of 0.95KJ/kgK. The pan is filled with 5kg water of density 1000kg/m3 and heat capacity of 4.2kL/kgK. If the pan and water are at room temperature of 20°C, how much energy is required to raise the temperature to the boiling point of water.arrow_forwardThe boiling temperature of nitrogen at atmospheric pressure at sea level (1 atm pressure) is -196 °C. Therefore, nitrogen is commonly used in low-temperature scientific studies since the temperature of liquid nitrogen in a tank open to the atmosphere will remain constant at -196 °C until it is depleted. Any heat transfer to the tank will result in the evaporation of some liquid nitrogen, which has a heat of vaporization of 198 kJ/kg and a density of 810 kg/m3 at 1 atm. Consider a 3-m-diameter spherical tank that is initially filled with liquid nitrogen at 1 atm and -196 °C. The tank is exposed to ambient air at 15° C, with a combined convection and radiation heat transfer coefficient of 35 W/m2⋅K. The temperature of the thin-shelled spherical tank is observed to be almost the same as the temperature of the nitrogen inside. Determine the rate of evaporation (in kg/s) of the liquid nitrogen in the tank as a result of the heat transfer from the ambient air if the tank is insulated with…arrow_forwardA 10 cm outer diameter pipe carrying saturated steam at a temperature of 195C is lagged to 20 cm diameter with magnesia and further lagged with laminated asbestos to 25 cm diameter. The entire pipe is further protected by a layer of canvas. If the temperature under the canvas is 20°C, find the mass of steam condensed in 8 hrs on a 100m length of pipe and interface temperature. Take thermal conductivity of magnesia as 0.07 W/m – K and that of asbestos as 0.082 W/m – k. Neglect the thermal conductivity of the pipe material. The latent heat of steam for given conditions can be taken as 1951 kJ/kg–K.arrow_forward
- A wall in a house contains a single window. The window consists of a single pane of glass whose area is 0.13 m2 and whose thickness is 8 mm. Treat the wall as a slab of the insulating material Styrofoam whose area and thickness are 19 m2 and 0.10 m, respectively. Heat is lost via conduction through the wall and the window. The temperature difference between the inside and outside is the same for the wall and the window. Of the total heat lost by the wall and the window, what is the percentage lost by the window?arrow_forwardA window panel that measures 20.0 cm by 15.0 cm is set into the front door of a house. The glass is 0.32 cm thick. The temperature outdoors is −15°C and inside is 22°C. 1-The single-paned window is replaced by a double-paned window with an argon gas gap of 12mm between the two panes. Then the double panes window replaced by Tripple gated window 3-then the Tripple gated window replaced by quadruple glazed window.The inner surface of the inner pane is at 22°C, and the outer surface of the outer pane is at −15°C. ( C glass=0.63 W/mK)Specific heat of Argon is 0.52 J/g K. Latent Heat of Fusion of Argon is 1.188 kJ/mol .calculat and compare the heat transfersarrow_forward(b) A hydrogen gas cylinder is situated in the cylinder cage. The cylinder wall is constructed from 15.5 mm carbon fiber (kcp = 0.75 W mK-¹). The outside of the cylinder is lagged with an inner 10 mm layer of ceramic insulation (kc = 0.08 W mK-¹) and an outer 80 mm layer of fiberglass insulation (kp = 0.15 W mK-¹). The temperature on the hydrogen gas is 150 °C and the temperature of the cylinder cage is 45 °C. Given that the walls of the cylinder can be assumed to be flat and neglecting the contribution of radiation, calculate: (i) the heat flux per square meter of the gas cylinder wall (ii) the temperature at the interface between the fibreglass and the ceramic insulation. 1.1arrow_forward
- A pipe 30 m long with an outer diameter of 75 mm is used to deliver steam at a rate of 2000 kg / hour. The vapor pressure is 198.53 kPa entering the pipe with a quality of 98%. The pipe needs to be insulated with a thermal conductivity of 0.2 W / (m K) so that the quality of the steam will only slightly decrease to 95%. The outer surface temperature of the insulation is assumed to be 25 ° C. Ignore resistance conductive of the pipe material and it is assumed that there is no pressure drop in the pipe. a. Determine the enthalpy of incoming vapor = AnswerkJ / kg. b. Determine the enthalpy of steam coming out = AnswerkJ / kg. c. Determine the vapor heat change / loss along the flow = Answerwatt. d. Specify the minimum required insulation thickness = Answercm.arrow_forwardA square thermal window is constructed of two sheets of flat glass, each 4.00 mm thick, separated by 5.00 mm of stationary air. If the inside of the window is at 20.0 oC, the external one at -30.0 oC and the cross-sectional area of the window is 6.00 m2, determine for the steady state: kglass= 0,80 W/(m*K); kair= 0,024 W(m*K) a) The temperatures between the air layer ?1 and ?2 indicated in the figure.b) The flow of heat through the windowarrow_forwardIf a 20 cm long and 0.4 mm diameter platinum wire is placed horizontally in a water container with a temperature of 35°C and the surface temperature is 90°C, calculate the heat lost by the wire.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY

Elements Of Electromagnetics
Mechanical Engineering
ISBN:9780190698614
Author:Sadiku, Matthew N. O.
Publisher:Oxford University Press

Mechanics of Materials (10th Edition)
Mechanical Engineering
ISBN:9780134319650
Author:Russell C. Hibbeler
Publisher:PEARSON

Thermodynamics: An Engineering Approach
Mechanical Engineering
ISBN:9781259822674
Author:Yunus A. Cengel Dr., Michael A. Boles
Publisher:McGraw-Hill Education

Control Systems Engineering
Mechanical Engineering
ISBN:9781118170519
Author:Norman S. Nise
Publisher:WILEY

Mechanics of Materials (MindTap Course List)
Mechanical Engineering
ISBN:9781337093347
Author:Barry J. Goodno, James M. Gere
Publisher:Cengage Learning

Engineering Mechanics: Statics
Mechanical Engineering
ISBN:9781118807330
Author:James L. Meriam, L. G. Kraige, J. N. Bolton
Publisher:WILEY
Heat Transfer – Conduction, Convection and Radiation; Author: NG Science;https://www.youtube.com/watch?v=Me60Ti0E_rY;License: Standard youtube license