
Concept explainers
Nitrobenzene has the skeleton
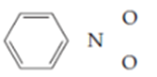
The number of available valence electrons is: from the phenyl group, ____; from the nitrogen atom, ____; and from each of two oxygen atoms, ____, for a total of ____. Filling in all single bonds and adding the appropriate unshared pairs gives
______________________________
The
______________________________
which has the correct number of electrons. In this structure the nitrogen atom is sharing ____ pairs of electrons. From each shared pair the nitrogen owns ____ electron for a total of ____. Nitrogen is a Group ____ element and would have outer shell electrons in the unbonded, neutral state. Since the nitrogen atom in nitrobenzene has one fewer electron than it would in the neutral state, it has a formal charge of +1 . This is added to the Lewis structure as + giving
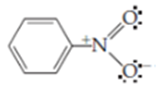
Since an electron is negatively charged, a shortage of one electron results in a single positive charge
Want to see the full answer?
Check out a sample textbook solution
Chapter 1 Solutions
Pushing Electrons
- The Lewis structure of acetone is Circling the carbonyl carbon, i.e., the carbon atom attached to oxygen, and its octet gives Circling the oxygen atom and its octet gives Thus, atoms share electrons in making bonds, and a pair of electrons may be included in the octet of two different atoms. When computing the formal charge on an atom, the number of electrons that belong to that atom is compared with the number of electrons the atom would have in the unbonded and neutral state. If the two numbers are the same, the formal charge on the atom is zero. In a Lewis structure both electrons in an unshared pair belong to the atom, and one of every pair of shared (bonding) electrons belongs to the atom.arrow_forwardExample of free radicals of H2O with excess acidsarrow_forward: : X: The Lewis representation above depicts a reaction between a halogen (blue) and a main-group element from group v (red). In this representation, each Y atom needs electron(s) to complete its octet, and gains these electrons by forming bond(s) with atoms of X. There are unshared electron pair(s) and bonding electron pair(s) in the product molecule. The bonds in the product arearrow_forward
- Make sure when you draw the Lewis structure you show which element has the 1- charge. Make sure the rest have the ideal # of bondsarrow_forwardIonic bonds also show unequal charges in bonding. However, due to the strong electronegativity of one atom, the e- will be pulled away leaving ___________ on the two atoms. This creates _____________. These are often created in water in organisms to carry out metabolic functions. Give an example: ______________arrow_forwardDraw the correc (best) Lewis structure (LS) for the ionic compound K2SeO3 (on looose leaf paper--NOT TO BE SUBMITTED) and then answer the following questions.(NOTE: Use the cardinal numbers 0, 1, 2, 3, and so on for any quantity required)a) What charge does the K ion have in this compound? (give size & sign of charge: e.g., 1+, 2-, etc.) b) What charge does the SeO3 ion have in this compound? (give size & sign of charge: e.g., 1+, 2-, etc.) c) How many lines, if any, attach each K ion to the SeO3 ion? d) How many valence electrons does the SeO3 ion (alone) have? e) How many single bonds does the LS of the SeO3 ion have? f) What is the number of lone electron pairs in the LS of the SeO3 ion?arrow_forward
- rn/takeAssignment/takeCovalentActivity.do?locator=Dassignment-take [Review Topics] [References] Use the References to access important values if needed for this question. The following Lewis diagram represents the valence electron configuration of a main-group element. This element is in group According to the octet rule, this element would be expected to form an ion with a charge of| If X is in period 4, the ion formed has the same electron configuration as the noble gas The symbol for the ion is M Submit Answer Retry Entire Group 8 more group attempts remaining M) M ts 2reg pts M) 1 pts OM) Previous Next Email Instructor Save and Exitarrow_forwardse the References to access important values if needed for this question. To answer the questions, interpret the following Lewis structure for CO3²-. :0: 0 Submit Answer :0: For the central carbon atom: The number of lone pairs The number of single bonds The number of double bonds = = Retry Entire Group 7 more group attempts remainingarrow_forwardAutoSave Module 4; Lewis Structures of Covalent Compounds - Saved to this PC P Searc Off File Home Insert Draw Design Layout References Mailings Review View Help Grammarly Times New Roma v 12 A A Aa v AaBbCcI AaBbCcI Aa Paste в IU ab х, х* А A I Normal I No Spac.. Hea Clipboard Font Paragraph 4..| 5 9... |·· 10. . |. 11. ..| 12. . | 13... 14. 15. * The speaker uses the term "lone electron pair" instead of non-bonding electron pair. It is the same thing. **Number VESPR Groups is equal to the number of atoms bonded to the central atom plus the mumber oaf ncn-bonding pairs. Draw the Lenis structure Name the shape. ihoning the correct :kape. LUse element symbols, dots for ncn- bonding electron pairs and dashes for banded electrons. BeCl, со. HCN BF, CH.O so, CH, NH, Number of VESPRarrow_forward
- Consider the following incomplete structures. On your rough sheet, complete their correct Lewis structures (including minimizing formal charge) by adding electrons/bonds where necessary. Which statement below is true of the completed Lewis structures? Hint: Consider the potential for multiple bonds on the molecules and be sure to account for all valence electrons. The NOCI," exhibits both formal charges and resonance hybrids, while the BrOCI," exhibits resonance hybrids but no formal chargés None of these. The NOCI, exhibits formal charges but no resonance hybrids, while the BrOCI," exhibits both formal charges and resonance hybrids The NOCI, exhibits both residual formal charges and resonance hybrids, while the BrOCI, exhibits formal charges but no resonance hybrids At least one atom in each molecule exhibits formal charges, and the molecules have no resonance hybrids The BrOCI, exhibits formal charges but no resonance hybrids, while the NOCI, exhibits resonance hybrids but no formal…arrow_forward*:Y:X: :X: The Lewis representation above depicts a reaction between a halogen (blue) and a main-group element from group (red). In this representation, each Y atom needs electron(s) to complete its octet, and gains these electrons by forming bond(s) with atoms of X. There are unshared electron pair(s) and bonding electron pair(s) in the product molecule. The bonds in the product arearrow_forwardcentral atom underlined: NH3O ICl3 XeO3 C4H4 O.R.-Lewis structure: Remember to always: • Show charge, if present. • Draw at least one more equivalent resonance structure (with «) if present, to describe delocalized covalent bonding. count all lone pairs in LS: polar: Y/N?arrow_forward
