
Concept explainers
(a)
Interpretation:
The reaction with detailed mechanism for the formation of the given compound from the corresponding
Concept introduction:
The electrophilic addition reaction of an alkene occurs through formation of a more stable carbocation. The electrophilic addition of water across the double bond in an acidic condition produces the alcohol product. The reaction proceeds with proton transfer reaction to form a stable carbocation, followed by the action of water as a nucleophile.
Answer to Problem 11.33P
The reaction equation and the detailed mechanism for the formation of
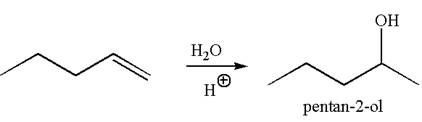
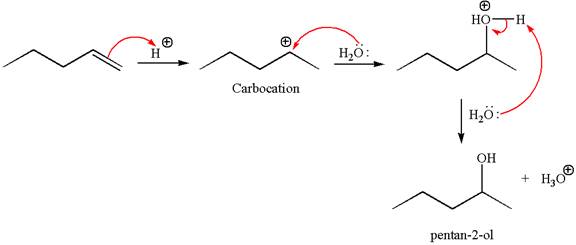
Explanation of Solution
The structure for the given compound
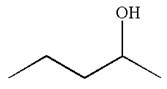
To form the given alcohol by electrophilic addition of water, the double bond must be between the carbon where the
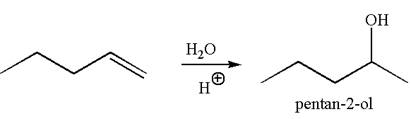
The reaction occurs in acidic condition. Thus the first step is the formation of a secondary carbocation by proton transfer reaction. The proton transfers to the less substituted carbon, forming a more stable carbocation.
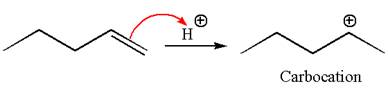
In the second step, the water molecule acts as a nucleophile and attacks the carbocation, forming the desired product, followed by deprotonation of the positively charged oxygen.
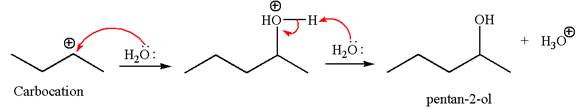
The reaction equation and detailed mechanism are drawn for the formation of
(b)
Interpretation:
The reaction with detailed mechanism for the formation of the given compound from the corresponding alkene by single electrophilic addition reaction is to be drawn.
Concept introduction:
The electrophilic addition reaction of an alkene occurs through formation of a more stable carbocation. The electrophilic addition of water across the double bond in an acidic condition produces the alcohol product. The reaction proceeds with proton transfer reaction to form a stable carbocation, followed by the action of water as a nucleophile.
Answer to Problem 11.33P
The reaction equation and the detailed mechanism for the formation of
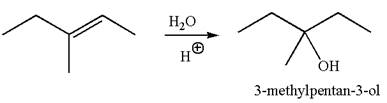
Explanation of Solution
The structure for the given compound
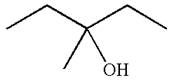
To form the given alcohol by electrophilic addition of water, the double bond must be between the carbon where the
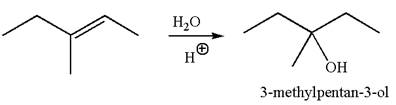
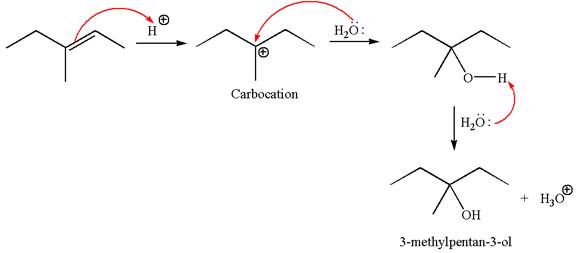
The reaction occurs in acidic condition. Thus the first step is the formation of a tertiary carbocation by proton transfer reaction. The proton transfers to the less substituted carbon, forming a more stable carbocation.
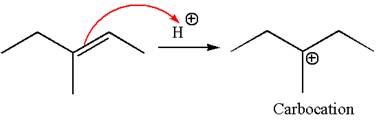
In the second step, the water molecule acts as a nucleophile and attacks the carbocation, forming the desired product, followed by deprotonation of the positively charged oxygen.
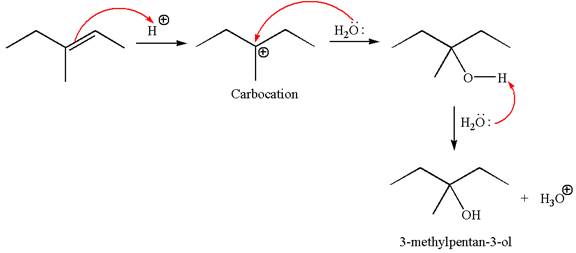
The reaction equation and detailed mechanism are drawn for the formation of
(c)
Interpretation:
The reaction with detailed mechanism for the formation of the given compound from the corresponding alkene by single electrophilic addition reaction is to be drawn.
Concept introduction:
The electrophilic addition reaction of an alkene occurs through formation of a more stable carbocation. The electrophilic addition of water across the double bond in an acidic condition produces the alcohol product. The reaction proceeds with proton transfer reaction to form a stable carbocation, followed by the action of water as a nucleophile.
Answer to Problem 11.33P
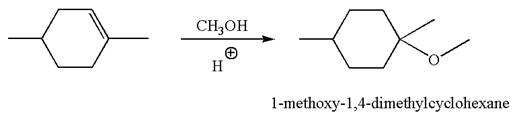
The reaction equation and the detailed mechanism for the formation of
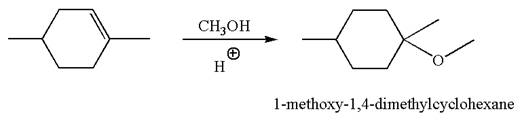
Explanation of Solution
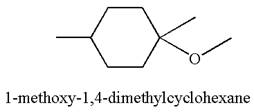
The structure for the given compound
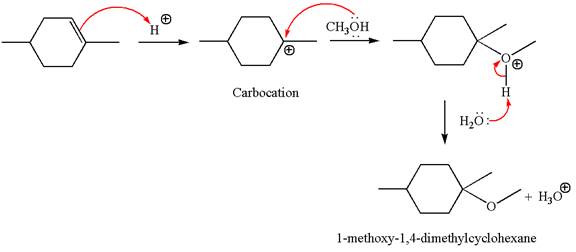
To form the given ether by electrophilic addition of alcohol, the double bond must be between the carbon where the
The reaction occurs in acidic condition. Thus the first step is the formation of a tertiary carbocation by proton transfer reaction. The proton transfers to the less substituted carbon, forming a more stable carbocation.
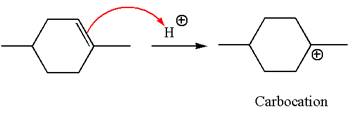
In the second step, the methanol molecule acts as a nucleophile and attacks the carbocation, forming the desired product, followed by deprotonation of the positively charged oxygen.
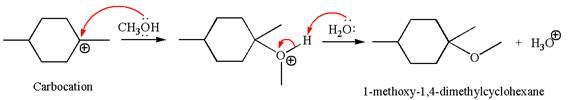
The reaction equation and detailed mechanism are drawn for the formation of
(d)
Interpretation:
The reaction with detailed mechanism for the formation of the given compound from the corresponding alkene by single electrophilic addition reaction is to be drawn.
Concept introduction:
The electrophilic addition reaction of an alkene occurs through formation of a more stable carbocation. The electrophilic addition of water across the double bond in an acidic condition produces the alcohol product. The reaction proceeds with proton transfer reaction to form a stable carbocation, followed by the action of water as a nucleophile.
Answer to Problem 11.33P
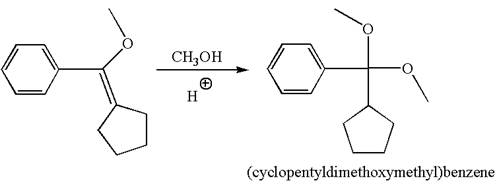
The reaction equation and the detailed mechanism for the formation of (cyclopentyldimethoxymethyl) benzene from alkene are
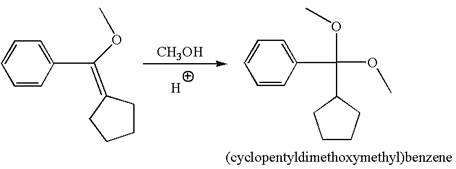
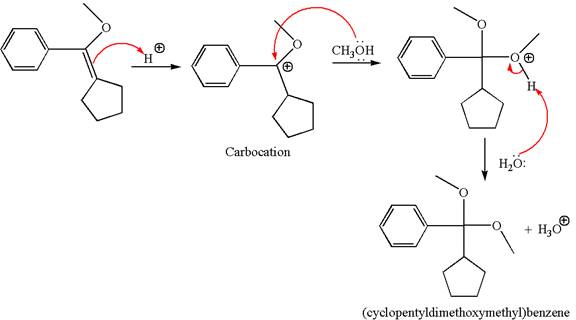
Explanation of Solution
The structure for the given compound (cyclopentyldimethoxymethyl) benzene is
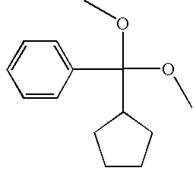
To form the given ether by single electrophilic addition of alcohol, the double bond must be between the carbon where the
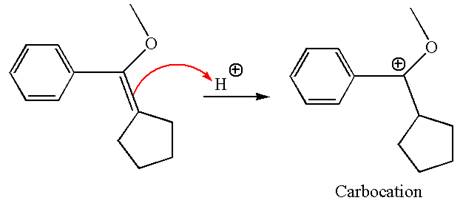
The reaction occurs in acidic condition. Thus the first step is the formation of a tertiary carbocation by proton transfer reaction. The proton transfers to the less substituted carbon, forming a more stable carbocation.
In the second step, the methanol molecule acts as a nucleophile and attacks the carbocation, forming the desired product, followed by deprotonation of the positively charged oxygen.
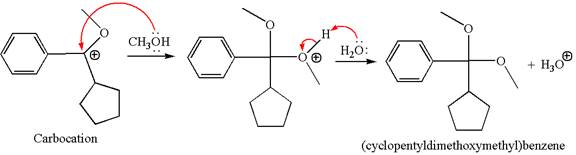
The reaction equation and detailed mechanism are drawn for the formation of (cyclopentyldimethoxymethyl) benzene by identifying the corresponding alkene.
Want to see more full solutions like this?
Chapter 11 Solutions
Organic Chemistry: Principles and Mechanisms (Second Edition)
- Draw the structure for each of the following molecules. (a) 1,3-divinylcyclohexane; (b) 3-allyl-4-vinylcyclopentene;(c) dimethylacetylene; (d) divinyl ether; (e) 4-vinylocta-1,3,7-triene; (f) 2-allylcyclohexa-1,3-dienearrow_forwardPredict the product for the reaction between m-ethylbenzoyl chloride and each of the following. Draw the complete, detailed mechanism for each reaction. If no reaction is expected to occur, write “no reaction." (a) NaOH, then H30*; (b) CH3NHLI; (c) CH;CH,OK; (d) C6H5CO,K; (e) CH3CI; (f) CH;OCH3 `CI m-Ethylbenzoyl chloridearrow_forwardDraw the structure of each of the following molecules. (a) (R)-3-chloropent-1-ene; (b) (2S,3S)-2-bromo-3-chloropentane; (c) (R)-1-bromo-(R)-2-iodocyclopentane; (d) (S)-3-chlorocyclohexene; (e) (1R,2S)-1,2-dibromocyclopentanearrow_forward
- Reaction of this bicycloalkene with bromine in carbon tetrachloride gives a trans dibromide. In both (a) and (b), the bromine atoms are trans to each other. However, only one of these products is formed. Which trans dibromide is formed? How do you account for the fact that it is formed to the exclusion of the other trans dibromide?arrow_forwardDraw the structure of each molecule. (a) (E)-4-carbamoylbut-3-enoic acid; (b) 3-carbamoylpentanediamide; (c) 4,6-dioxohexanenitrile; (d) (1S,2S)-2-methoxycyclopent-3-ene-1-carbonitrile; (e) cyclohexa-3,6-diene-1,3-dicarboxylic acidarrow_forwardPlease explain, thank you! Each of the following compounds can be produced from an alkene, using a single electrophillic addition reaction. Write that reaction and draw its complete, detailed mechanism. (a) pentan-2-ol (b) 3-methylpentan-3-ol (c) 1-methoxy-1,4-dimethylcyclohexane (d)(cyclopentyldimethoxymethyl)benzenearrow_forward
- Consider the reaction scheme shown below. HCI [1] ОН (a) Provide a detailed mechanism for reaction [1].arrow_forwardFor each of the following reactions, draw a complete, detailed mechanism and predict the major organic products. (a) 2-Methylbut-2-ene Br2 ? CCI4 (b) 2-Methylbut-2-ene ? H2O Br2 (c) Неха-1,5-diene Cl2 (excess) ? CCI4 (d) 3-Ethylpent-1-yne Br2 (excess) ? CCI4arrow_forwardThe following isomers react separately with sodium hydroxide to give different products with the formulas shown. HO. .CI Но NaOH NaOH C3H1,02 (a) Draw the structure of each product. (b) Draw the mechanism that accounts for the formation of each of those products. (c) Explain why the isomeric reactants lead to different products.arrow_forward
- Complete the following reactions, clearly indicating regio-and stereochemistry where applicable. In cases, where ortho-and para-products are formed, draw both.arrow_forwardPredict the product for the reaction between methyl benzoate and each of the following. If no reaction is expected to occur, write “no reaction.” For those reactions that do occur, draw the complete, detailed mechanism. (a) LiAlH4, then H3O+; (b) CH3CH2CH2Li (excess), then H3O+; (c) (CH3CH2)2CuLi; (d) C6H5MgBr (excess), then H+; (e) DIBAH, then H3O+arrow_forwardThere are two isomeric cyclohexa-1,4-diene products when toluene undergoes the Birch reduction (see Problem 25.24). (a) Draw the mechanism that leads to the formation of the major product. (b) Will the Birchreduction of toluene occur faster or slower than the Birch reduction of benzene itself? Hint: Is –CH3 an electron-donating or an electron-withdrawing group?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
