
Interpretation: Structures of the possible isomeric heptanes should be drawn with names.
Concept introduction: Molecules that possess identical molecular formula but vary in the manner of their connectivity of the bonds are referred as structural isomers. These are essentially found in
For instance, smallest branched alkane possible is
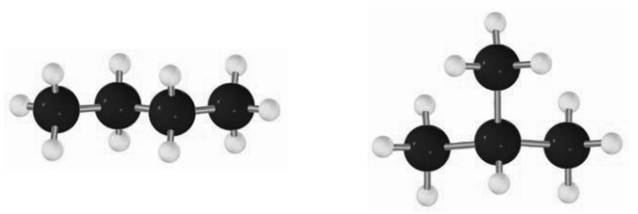
The two compounds infact constitute a pair of constitutional isomers. These kinds of isomers are formed by cleavage and replacement of bonds unlike the conformational isomers that results due to rotation around the bond.
As per IUPAC recommendations longest chain found in a continuous manner in a branched molecule is chosen as parent chain.
The IUPAC name begins with prefix to designate the number of
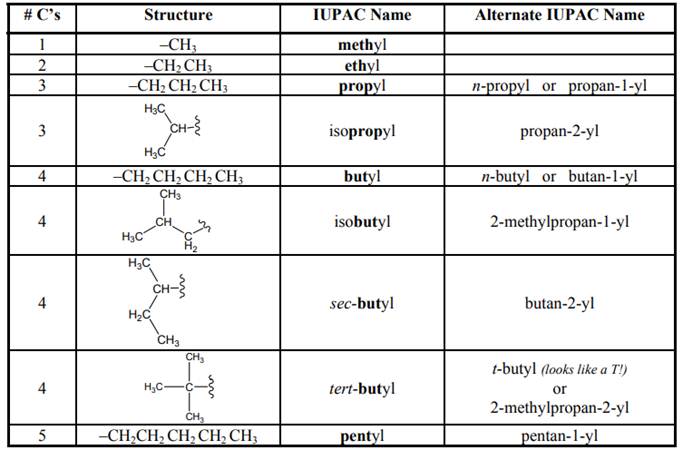
Names of branches that occur commonly as side chains are listed below:
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Organic Chemistry: Structure and Function
- Write structures for the three isomers of the aromatic hydrocarbon xylene, C6H4(CH3)2.arrow_forwardSummarize the nomenclature rules for alkanes, alkenes, alkynes, and aromatic compounds. Correct the following false statements regarding nomenclature of hydrocarbons. a. The root name for a hydrocarbon is based on the shortest continuous chain of carbon atoms. b. The suffix used to name all hydrocarbons is -ane. c. Substituent groups are numbered so as to give the largest numbers possible. d. No number is required to indicate the positions of double or triple bonds in alkenes and alkynes. e. Substituent groups get the lowest number possible in alkenes and alkynes. f. The ortho- term in aromatic hydrocarbons indicates the presence of two substituent groups bonded to carbon- 1 and carbon-3 in benzene.arrow_forwardHow does the structure of a cycloalkane differ from that of a straight-chain or branched-chain alkane?arrow_forward
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax
Chemistry by OpenStax (2015-05-04)ChemistryISBN:9781938168390Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark BlaserPublisher:OpenStax Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning





