
Concept explainers
(a)
Interpretation: Structures of the five isomeric hexanes should be drawn.
Concept introduction: Molecules that possess identical molecular formula but vary in the manner of their connectivity of the bonds are referred as structural isomers. These are essentially found in
For instance, smallest branched alkane possible is
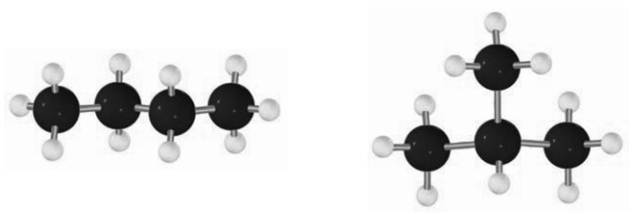
The two compounds infact constitute a pair of constitutional isomers. These kinds of isomers are formed by cleavage and replacement of bonds, unlike the conformational isomers that result due to rotation around the bond.
(b)
Interpretation: Structures of all the possible next higher and lower homologs of
Concept introduction: Each member of a particular
Want to see the full answer?
Check out a sample textbook solution
Chapter 2 Solutions
Organic Chemistry: Structure and Function
- (a) What is the molecular formula of hexane, the alkane withsix carbonsarrow_forwardDraw the structures for (a) 2-methyl-1,3,5-hexatriene and (b) 1,6-dimethoxyhexa-1,5-diene.arrow_forwardThe condensed structure of geraniol is shown. (E)-geraniol is the stereoisomer responsible for how roses smell. (a) Draw the line structure of (E)-geraniol.arrow_forward
- 1. (A) DRAW are all the possible isomers for dibromobutane and the cylic isomers for C3H4Br2 (B) WRITE THE STRUCTURAL OR CONDENSED FORMULAR FOR C3H7Br C3H6Br2 C3H5Br3 C5H11Brarrow_forwardThe meiting points and boiling points of two isomeric alkanes are as follows: CH3(CH2);CH3, mp = -57 °C and bp = 126 °C; (CHalsCC(CH3)3, mp = 102 °C and bp = 106 °C. (a) Explain why one isomer has a lower melting point but higher boiling point. (b) Explain why there is a small difference in the boiling points of the two compounds, but a huge difference in their melting points.arrow_forwardGiven each of the IUPAC names provided, draw the corresponding structure. (a) 2,2,4-trimethylpentane;(b) 3-ethyl-2,3-dimethylpentane; (c) 2,2,3,3-tetramethylhexanearrow_forward
- (a) Write a chemical equation involving structural formulas for the catalytic cracking of decane into an alkaneand an alkene that contain equal numbers of carbonatoms. Assume that both products have straight chainsof carbon atoms.(b) Draw and name one other isomer of the alkene.arrow_forwardAn alkane, P, has the molecular formula, C,H.. An alkene, Q, has the molecular formula, C H,. (a) Name P and Q ánd write their full structural formulae. (b) State two differences between P and Q in terms of their structures. x'arrow_forward(a) Draw and name all five isomers of formula C3H5F.(b) Draw all 12 acyclic (no rings) isomers of formula C4H7Br. Include stereoisomers.arrow_forward
- Draw structures for the following haloalkanes. (a) 1,2,3-tribromohexane; (b) 2,2,3,3,4-pentachlorohexane;(c) 1,2-dichloro-4-nitrohexane; (d) 1,2-dichloro-3-methoxycyclopentanearrow_forwardDraw structures for the following molecules. (a) 2-iodo-2,3-dimethylheptane (b) 4-cyclopropyl-3-ethyl-2-methyloctane (c) 1-ethyl-2,4-dimethylcyclopentanearrow_forward5) 1-butene and cyclobutane are constitutional isomers; explain why this is true. What are the remaining three possible isomers? Draw the structures.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





