
Organic Chemistry: Structure and Function
8th Edition
ISBN: 9781319079451
Author: K. Peter C. Vollhardt, Neil E. Schore
Publisher: W. H. Freeman
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 2, Problem 65P
Interpretation Introduction
Interpretation: The kind of Newman projection of the conformer of butane shown in below structural representation should be identified.
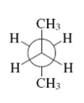
Concept introduction: Various interconvertible forms that results from rotation around the
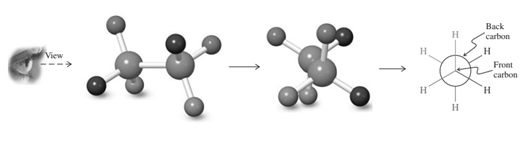
Thus in Newman's projection of simple ethane molecule, the “front”
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Ethane spends all its time in the staggered conformation because it has the lowest potential energy.
a. True
b. False
c. Neither
Look at the model of chloroethane. a) Do all chloroethane molecules spend all of their time in this preferred conformation? Explain why or why not. b) What is the preferred conformation called?
Consider rotation around the carbon–carbon bond in 1,2-dichloroethane (ClCH2CH2Cl).
a.Using Newman projections, draw all of the staggered and eclipsed conformations that result from rotation around this bond.
b.Graph energy versus dihedral angle for rotation around this bond.
Chapter 2 Solutions
Organic Chemistry: Structure and Function
Ch. 2.1 - Prob. 2.1ECh. 2.1 - Prob. 2.2ECh. 2.1 - Prob. 2.3ECh. 2.1 - Prob. 2.5TIYCh. 2.1 - Prob. 2.6ECh. 2.1 - Prob. 2.7ECh. 2.2 - Prob. 2.8ECh. 2.3 - Prob. 2.9ECh. 2.3 - Prob. 2.10ECh. 2.3 - Prob. 2.11E
Ch. 2.3 - Prob. 2.12ECh. 2.3 - Prob. 2.13ECh. 2.3 - Prob. 2.14ECh. 2.3 - Prob. 2.15ECh. 2.3 - Prob. 2.17TIYCh. 2.3 - Prob. 2.19TIYCh. 2.5 - Prob. 2.20ECh. 2.6 - Prob. 2.21ECh. 2.6 - Prob. 2.22ECh. 2.6 - Prob. 2.23ECh. 2.6 - Prob. 2.25TIYCh. 2.7 - Prob. 2.26ECh. 2.9 - Prob. 2.28TIYCh. 2 - Prob. 31PCh. 2 - Prob. 32PCh. 2 - Prob. 33PCh. 2 - Prob. 34PCh. 2 - Prob. 35PCh. 2 - Prob. 36PCh. 2 - Prob. 37PCh. 2 - Prob. 38PCh. 2 - Prob. 39PCh. 2 - Prob. 40PCh. 2 - Prob. 41PCh. 2 - Prob. 42PCh. 2 - Prob. 43PCh. 2 - Prob. 44PCh. 2 - Prob. 45PCh. 2 - Prob. 46PCh. 2 - Prob. 47PCh. 2 - Prob. 48PCh. 2 - Prob. 49PCh. 2 - Prob. 50PCh. 2 - Prob. 51PCh. 2 - Prob. 52PCh. 2 - Prob. 53PCh. 2 - Prob. 54PCh. 2 - Prob. 55PCh. 2 - Prob. 56PCh. 2 - Prob. 57PCh. 2 - Prob. 58PCh. 2 - Prob. 59PCh. 2 - Prob. 60PCh. 2 - Prob. 61PCh. 2 - Prob. 62PCh. 2 - Prob. 63PCh. 2 - Prob. 64PCh. 2 - Prob. 65PCh. 2 - Prob. 66P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- a. Draw the lowest energy conformation for the compound shown in the image. b. Draw the complete structural formula of the following compound: cis-1-bromo-3-chlorocyclohexane.arrow_forwardConsider 1,2-dimethylcyclohexane.a. Draw structures for the cis and trans isomers using a hexagon for the six-membered ring.b. Draw the two possible chair conformations for the cis isomer. Which conformation, if either, is more stable?c. Draw the two possible chair conformations for the trans isomer. Which conformation, if either, is more stable?d. Which isomer, cis or trans, is more stable and why?arrow_forward• Draw the chair conformations of the following compounds and determine which geometric isomer is more stable. Also show using Newman projection that one conformation is more stable than the other. a. cis-1-isopropyl-2-methylcyclohexane b. 1-methycyclohexanearrow_forward
- The dimethylcyclohexane with the structure shown below is: ÇH3 CH3 Select one: O a. a trans isomer with the CH3 groups in axial positions. O b. a cis isomer with the CH3 groups in equatorial positions. O c. a cis isomer with the CH3 groups in equatorial and axial positions. O d. a trans isomer with the CH3 groups in equatorial positions.arrow_forwardModel 2. Cyclohexane Chair Conformation Another helpful way to drawn a non-planar example of cyclohexane is to draw its most stable (lowest energy) conformation called a chair conformation. III 8. Build two models of cyclohexane. The chair conformation is the most stable conformation of cyclohexane because all of the bond angles around the carbon atoms are at 109.5ª and all carbon atoms are in the more stable staggered conformation. Cyclohexane is the only cyclic structure that allows all the bond angles around each carbon to be 109.5", thus is the only ring that adopts a true chair conformation. Form the chair conformation with both of your cyclohexane models. Look down each carbon carbon bond and verify that all carbons are in a staggered conformation. 9. 10. Because of this conformation, there are two types of positions a group can occupy when it is attached to a cyclohexane chair, either axial (A) or equatorial (E). 11. Axial hydrogens alternate straight up and straight down as we…arrow_forwardC. Exercises 1. Which molecule representation best shows the bond interactions? a. Wedge and dash b. Sawhorse c. Newman d. Fischer Which molecule representation best combines the 3D molecule and the bond interactions? a. Wedge and dash b. Sawhorse c. Newman d. Fischer 3. Which molecule representation best showws the 3D model? a. Wedge and dash b. Sawhorse c. Newman d. Fischer 4. Staggered and eclipsed conformations are best shown using which projection? a. Wedge and dash b. Sawhorse c. Newman d. Fischer 5. Which is a more stable conformation? a. Eclipsed ethane b. Staggered ethanearrow_forward
- Draw a chair conformation of cyclohexane with one CH3CH2 group and one CH3 group that fits each description. a.a 1,1-disubstituted cyclohexane with an axial CH3CH2 group b. a cis-1,2-disubstituted cyclohexane with an axial CH3 group c. a trans-1,3-disubstituted cyclohexane with an equatorial CH3 group d. a trans-1,4-disubstituted cyclohexane with an equatorial CH3CH2 group.arrow_forwardDraw a chair conformation of cyclohexane with one CH3CH2 group and one CH3 group that fits each description. a. a 1,1-disubstituted cyclohexane with an axial CH3CH2 group b. a cis-1,2-disubstituted cyclohexane with an axial CH3 group c. a trans-1,3-disubstituted cyclohexane with an equatorial CH3 group d. a trans-1,4-disubstituted cyclohexane with an equatorial CH3CH2 grouparrow_forward1: Draw the chain conformer of... 1a. Cyclohexane, label all the axial and equatorial hydrogens 1b. The most stable conformer of ethylcyclohexane 1c. The most sable conformer of trans-1-tert-butyl-3-methylcyclohexanearrow_forward
- 41. Draw the structure of a hydrocarbon that has six carbon atoms and a. three vinylic hydrogens and two allylic hydrogens. b. three vinylic hydrogens and one allylic hydrogen. c. three vinylic hydrogens and no allylic hydrogens.arrow_forward1,2-ditert-butylcyclohexane is an interesting case of a conformational study. The cis isomer is more stable than the trans isomer, although the trans isomer presents a conformer with both substituents in equatorial positions. In order to analyze this fact:a. Draw the conformational equilibrium of both compounds. Explain using and drawing the structures why this difference happens (the one described above). b. In the trans isomer, the di-axial conformation is 25.9 kJ / mol more stable than the di-equatorial. Calculate the energy of the di-equatorial conformation.arrow_forwardQUESTION 2 In the lowest energy chair conformation of cis-1,3-dimethylcyclohexane, how many axial positions are occupied by hydrogen atoms? A. 2 B. 3 C. 4 D. 6arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Chapter 4 Alkanes and Cycloalkanes Lesson 2; Author: Linda Hanson;https://www.youtube.com/watch?v=AL_CM_Btef4;License: Standard YouTube License, CC-BY
Chapter 4 Alkanes and Cycloalkanes Lesson 1; Author: Linda Hanson;https://www.youtube.com/watch?v=PPIa6EHJMJw;License: Standard Youtube License